Abstract
Development of the mammalian urogenital system requires a balance between survival and programmed cell death. Pro-survival molecules are crucial in preserving metanephric mesenchyme viability, and thus allowing nephrogenesis to proceed. At the same time, localized areas of apoptosis mediated by effector caspases are required for the appropriate morphogenesis of the kidney and urinary tract. Activation of the intrinsic pathway of apoptosis seems to be fundamental to the progression of cell death necessary to aid ureteric bud branching, nephrogenesis, and ureter-bladder connection. Here, we review what is currently known about survival and apoptosis in building functional kidneys and urinary tracts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Morphogenesis of most–if not all–organ systems involves a balance between cell survival and programmed cell death. While this has been well characterized in the development of certain structures, notably the limb and the nervous system, our understanding of this balancing act in other organs has lagged. The vertebrate urogenital system (UGS) is one such example.
In mammals, development of the UGS proceeds through two transient structures, the pro- and mesonephros, before forming the metanephros (reviewed in [1]). The pronephros initiates from the intermediate mesoderm, which epithelializes to form the Wolffian duct. This duct elongates caudally along the body axis, passing through a transient mesonephros stage wherein mesonephric tubules are induced from the adjacent mesenchyme. The Wolffian duct serves as the basis of reproductive tract development; in males, it differentiates to form the epididymis and vas deferens, whereas in females, it guides the descent of the Müllerian duct, which is the precursor of the oviduct, uterus, and upper vagina. Sexual determination occurs when secreted signals result in apoptotic regression of either the Wolffian or Müllerian ducts [2].
Elongation of the Wolffian duct progresses until it fuses with the cloaca, the primordium of the bladder, and urethra. Subsequently, a diverticulum of the Wolffian duct, known as the ureteric bud, invades the metanephric mesenchyme. Reciprocal signaling between these compartments induces iterative rounds of ureteric bud branching and differentiation of the metanephric mesenchyme to give rise to the collecting ducts and nephrons of the adult kidney [3, 4]. It is interesting to note that in the advent of disrupted signaling between the metanephric mesenchyme and the ureteric bud, the default route for the mesenchymal cells is apoptosis, resulting in kidney agenesis [5]. Conversely, a normal level of apoptosis occurs among the metanephric mesenchyme during nephrogenesis, presumably aiding in morphogenesis [6].
Concomitant with the development of the kidney, the section of the ureteric bud outside the metanephric mesenchyme elongates and becomes coated with smooth muscle to form the ureter, which conducts urine from the functional kidney to the bladder. In order for the ureter to insert in the bladder at the appropriate junction it must separate from the Wolffian duct in a process known as distal ureter maturation [7, 8]. It was initially believed that the ureter was displaced to its final place in the bladder wall by the engulfment of the section of the nephric duct common to both ureter and Wolffian duct (common nephric duct, CND) into the bladder trigone. Recent advances in mouse models however, have shown that this process involves apoptosis of the CND and part of the distal ureter [7–9].
Apoptosis is the blanket term used to indicate cell death that is characterized by the orderly dismantling and packaging of cellular contents, followed by engulfment of the apoptotic bodies by phagocytes. This order of events results in well-defined morphological changes, including membrane blebbing, cell shrinkage, phosphatidyl serine exposure, nuclear condensation, and DNA fragmentation [10]. A wide variety of normal and pathological stimuli have been identified that initiate apoptosis, although entry into apoptotic pathways varies widely between them. There are two classically recognized entry points to the apoptotic cascade, namely, the intrinsic and the extrinsic pathways.
The intrinsic (mitochondrial-mediated) pathway of apoptosis is regulated through complex interactions between the pro- and anti-apoptotic members of the Bcl2-family of proteins. The anti-apoptotic family members, including Bcl2 itself, prevent programmed cell death by inhibiting the pro-apoptotic Bcl2 family proteins. These pro-death members are subdivided into both effector (Bak, Bax) and BH3-only proteins, which induce cell death by interacting either with the anti-apoptotic Bcl2 members or with both the anti-apoptotic and effector proteins [11, 12]. Upon stimulation of apoptosis, Bak and Bax oligomerize to open pores within the outer mitochondrial membrane (mitochondrial outer membrane permeabilization, MOMP) and release proteins, such as cytochrome c, apoptosis-inducing factor (AIF), and EndoG [13]. Diffusion of these soluble proteins from the mitochondrial intermembrane space to the cytosol is responsible for the majority of morphological changes undergone during apoptosis. AIF and EndoG are believed to mediate nuclear condensation and DNA fragmentation directly, in a caspase-independent manner [10, 14]. Cytochrome c, on the other hand, acts through effector caspases 3, 6, and 7 to induce membrane blebbing, cell shrinkage, and nuclear dismantling [15]. The effector caspase cascade is activated through the cleavage of procaspase-3 by the apoptosome, an association of apoptotic protease activating factor-1 (APAF-1) with cytochrome c and the initiator caspase-9 [16, 17]. Although MOMP occurs at variable lengths of time after proapoptotic stress, caspase activation and the ensuing morphological changes occur within minutes of mitochondrial dysfunction, making this a rapid cellular process during mammalian development [13].
The extrinsic (receptor-mediated) pathway of apoptosis is initiated when a death receptor [Fas, tumor necrosis factor (TNF) receptors] is bound by its ligand, which then recruits adaptor proteins and pro-caspase 8 to form the death-inducing signaling complex (DISC) [18]. Pro-caspase 8 is activated by auto-processing within the DISC to release the initiator caspase form, which can then either cleave the executioner caspase 3 directly or cleave the cytosolic BH3-only protein Bid to its activated tBid form [18, 19]. Interaction of tBid with Bax or Bak on the outer mitochondrial membrane results in MOMP releasing pro-apoptotic proteins from the intermembrane space and the coalescence of a branch of the extrinsic pathway with the intrinsic pathway of apoptosis [19].
The role that apoptosis plays in shaping tissue development can generally be considered in one of two lights: cell death may be the result of inductive signals, either autonomously by the cell or from an adjacent cell population, that activate the apoptotic pathways to eliminate unwanted cells. Conversely, apoptosis can be considered to be the default pathway for some cells that are preserved from death by survival factors. Here we discuss the role that apoptosis, or a lack thereof, plays in the development of the mammalian urogenital system and also highlight the defects that result from upsetting the apoptotic balance.
Survival in the UGS
Studies on the fate of cells during metanephric development revealed that metanephric mesenchyme not induced to transition to epithelia underwent apoptosis instead. Furthermore, these cells could be rescued from this fate in vitro by incubation with the embryonic spinal cord, thus suggesting that the mesenchyme is programmed for cell death and must be actively prevented from dying in order for nephrogenesis to proceed [6, 20]. An obvious candidate to provide the necessary pro-survival signals is the ureteric bud, which is believed to rescue the metanephric mesenchyme from death and then induce nephrogenesis. Initial attempts to prevent apoptosis of cultured metanephric mesenchyme identified epidermal growth factor (EGF) and protein kinase C (PKC) activators as promoting survival, indicating the existence of pro-survival kinase signaling pathways in metanephric mesenchyme cells [20, 21]. Indeed, further culture of metanephric mesenchyme in ureteric bud-conditioned media has demonstrated that ureteric bud cells secrete multiple soluble factors which rescue the mesenchymal cells from apoptosis, including basic Fgf/Fgf2 (Fig. 1a) [22, 23]. It is noteworthy, however, that Fgf2-deficient mice do not exhibit those defects in nephrogenesis, which would be expected were Fgf2 to be the only mesenchymal survival factor [24]. Unsurprisingly then, especially given the functional redundancy among family members, it has been demonstrated that Fgf8 also functions to prevent apoptosis in cultured metanephric mesenchyme (Fig. 1a) [25, 26]. Furthermore, conditional deletion of Fgf8 in the mouse metanephric mesenchyme results in aberrant apoptosis of nephron progenitors at the periphery of the developing kidney, halting nephrogenesis and leading to renal hypoplasia [25, 26]. In light of these findings, it is possible that the protective effect of Fgf2 observed in culture mimics the physiological role of Fgf8 during morphogenesis.
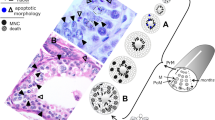
Survival and apoptosis signaling are needed for the development of the mammalian kidney and urinary tract. a Pro-survival signaling (green), mediated through the indicated genes, is necessary for the morphogenesis of the Wolffian duct, ureteric bud, metanephric mesenchyme, and all stages of nephrogenesis. b Induction of apoptosis (blue) by the indicated genes is required in regression of the mesonephric tubules, elimination of the common nephric duct, and in the metanephric mesenchyme as well as later stages of nephrogenesis. Cl Cloaca, CND common nephric duct, MM metanephric mesenchyme, MT mesonephric tubules, UB ureteric bud, WD Wolffian duct
Additional evidence that secreted fibroblast growth factors (Fgfs) initiate pro-survival signaling in nephron progenitors is obtained from mice in which Fgf receptors 1 and 2 (Fgfr1/2) are conditionally deleted in the metanephric mesenchyme. Although neither single Fgf receptor knockout exhibits defects in nephrogenesis, Fgfr1;Fgfr2 double mutant mesenchyme fails to condense around the branching ureteric bud primarily due to increased apoptosis [27]. Noting the earlier onset of aberrant apoptosis in the Fgf receptor double knockout mice as compared to the Fgf ligand knockouts suggests further redundancy in survival signaling in metanephric mesenchyme, likely through other Fgf family members. Several reports have described anti-apoptotic activities for other pathways, such as bone morphogenetic protein (Bmp) signaling, primarily by Bmp4 and Bmp7, during nephron differentiation [28, 29]. As the pro-survival effect of either of these two Bmps within the metanephric mesenchyme is weaker than that of the Fgfs, it is thought that the signaling potentiated through Bmps is complementary to that through the Fgfs [30]. Furthermore, while Bmp7 is indeed expressed in the nephrogenic precursors [29], Bmp4 acts on nephron precursors from the stromal mesenchyme (Fig. 1a) [28], lending credence to the notion of complementarity among Bmp family members in differentiating nephrons.
Wt-1 is another pro-survival transcription factor expressed early in the metanephric mesenchyme (Fig. 1a) [30]. Ablation of Wt-1 in mice is associated with increased apoptosis of the metanephric blastema accompanied by failure to induce the ureteric bud [31]. Unlike many other knockout models, lack of the ureteric bud is not the primary cause of apoptosis in the mesenchyme; rather, the defect is intrinsic to the metanephric mesenchyme as it is not rescued in recombination experiments [31]. Interestingly, Wt-1 morpholino-treated kidney explants recapitulated the apoptosis observed in vivo and further demonstrated a decrease in expression of Bmp7 and Pax2, both of which show aberrant apoptosis in loss-of-function models [32].
In addition to survival of the metanephric mesenchyme, preservation of the mesonephric lineages must also be considered (Fig. 1a). Misregulation of Wolffian duct extension leads to fulminant apoptosis, resulting in kidney and ureter agenesis as well as defects in reproductive tract development [33]. Pax2 and Pax8 have been implicated as the earliest regulators of this process, although the loss of both in the intermediate mesoderm results in apoptosis only after a delay of nearly 1 day [33]. The late onset of apoptosis suggests an indirect role of Pax genes in controlling survival, perhaps through the transcriptional activation of pro-survival molecules or inhibitors of apoptosis, rather than a direct impact on the apoptotic cascade itself. Interestingly, the region of apoptosis extends beyond the Pax2/8 expression domain, suggesting possible non-cell-autonomous control of adjacent mesodermal survival [33]. In addition, transgenic mice targeting the expression of pro-apoptotic Bax under the control of the Pax2 promoter have demonstrated that aberrant apoptotic activity in the Wolffian duct, such as that caused by the loss of Pax2/8, is sufficient to abolish kidney development [34].
Crucially, it seems that the dosage of Pax genes is important in determining the extent of apoptosis in the kidney; Pax2 heterozygote mice exhibit only a moderate increase in apoptosis, primarily along the branching ureteric bud, which disrupts collecting duct arborization and the rate of nephron formation [33, 35]. Concomitant loss of one Pax8 allele strengthens the Pax2 heterozygote phenotype and further sensitizes the nephrons to apoptosis as Pax8 is turned on at the renal vesicle stage [36]. Interestingly, suppression of apoptosis in Pax2 heterozygote mice by expression of the pro-survival molecule Bcl2 in the ureter and collecting ducts was able to rescue the hypoplastic kidney phenotype [37], suggesting that a large part of the defects of Pax2 heterozygous kidneys stem from ureter cell death. However, no changes in endogenous Bcl2 levels were originally noted in the ureters of Pax2 heterozygous mice, indicating that Pax2 protects ureter tip cells through alternative pro-survival molecules. In this respect, in murine collecting duct cells, Pax2 was demonstrated to activate transcription of neuronal apoptosis inhibitor protein (Naip), an endogenous inhibitor of caspase 3 and 7 expressed in the developing kidney [38].
The aforementioned pro-survival molecules focus for the most part on transcription factors which have an indirect role in controlling apoptosis; few studies have identified changes in apoptotic regulators that impact on kidney morphogenesis, perhaps due to the multiple levels of compensation that exist both among programmed cell death pathways and among induction and growth of the kidney. The foremost exception to this is Bcl2, which is expressed in both the ureteric bud and condensing mesenchyme during early kidney development (Fig. 1a) [39]. Surprisingly, upon the loss of both alleles, an increase in apoptosis is only observed in the metanephric blastema, resulting in fewer nephrons and a smaller nephrogenic zone, which is ultimately associated with mild renal hypoplasia and cystogenesis [39, 40]. Rescue of this phenotype is accomplished either through the retention of one allele of Bcl2 or through the concomitant loss of one allele of the pro-apoptotic gene Bim [41], thus suggesting that the main pro-survival action of Bcl2 in the kidney revolves around control of the mitochondrial pathway of apoptosis. Recently, an alternative, though not mutually exclusive, requirement for Bcl2 in nephrogenesis has been expounded in which Bcl2 protects migrating mesenchymal cells from anoikis, a form of apoptosis induced by cell detachment. It is proposed that by interacting with paxillin and focal adhesion kinase (FAK), Bcl2 is able to bypass integrin-mediated survival signals and circumvent the need for mesenchymal cell adhesion, thus allowing the cell movements required for nephrogenesis (Fig. 1a) [42, 43]. Accordingly, disrupting the interaction between paxillin and Bcl2 in embryonic kidney culture inhibited kidney morphogenesis and was accompanied by a moderate increase in apoptosis [42, 43].
It is worth remarking on the congruence seen among the pro-survival molecules required for kidney morphogenesis, in that there seems to be a network of transcription factors which regulate anti-apoptotic factors, such as Bcl2, through various means. The similarity of Bcl2 to other anti-apoptotic family members begs the question of whether these proteins also serve a pro-survival function in the kidney, either singly or in combination, that has not yet been revealed in mouse models. Furthermore, it is possible that the various actions of these pro-survival molecules serve to inhibit the activity of pro-apoptotic proteins in the kidney. In addition to the outcome of pro-survival signaling, it remains to be discovered how these signals are initiated and regulated. In light of the complementary actions of the secreted Fgfs and Bmps with the transcription factors Wt-1 and Pax2/8, it is tempting to postulate a linear cascade; however, given the precise requirements to prevent apoptosis, these processes are more likely to resemble a web of interactions. That these pro-survival pathways provide such redundancy in preserving specific cell populations underlines the delicate balance between survival and death during morphogenesis of the kidney.
Death in the UGS
Studies on the requirement for programmed cell death in kidney morphogenesis have been largely descriptive, with few molecular mechanisms identified. Apoptosis is often judged from activated caspase 3 staining, or nuclear fragmentation, which generally does not discriminate between the intrinsic and extrinsic apoptotic pathways. Furthermore, although apoptosis plays an undeniable role in sculpting the functional kidney, estimates of the prevalence of cell death at embryonic day (E) 16.5 of mouse kidney organogenesis vary from 0.3 to 3% of cells in the developing cortex [21, 44]. This has led to some confusion on exactly how important death is in the general scheme of kidney development. Regardless, apoptosis has been observed in virtually every compartment of the urogenital system throughout development, starting with regression of the pro- and mesonephros in females and continuing until a fully functional adult kidney is produced (Fig. 1b).
In order to give rise to the sexual dimorphism evident in the adult reproductive tracts, secreted signals at the sexually ambiguous embryonic stages containing both the Wolffian and Müllerian ducts induce the apoptotic degradation of either the former (in females) or the latter (in males). The choice between sexes is determined by the presence or absence of Müllerian inhibiting substance (MIS), which, when present in male embryos, signals through its receptors to promote Bmp signaling, activation of SMADs and, ultimately, apoptosis of the Müllerian duct (reviewed in [2]). In the absence of MIS, the Müllerian duct is maintained, whereas the Wolffian duct degenerates, possibly through the pro-apoptotic transcriptional activity of Msx2 [45]. This regression of the Wolffian and Müllerian ducts in mammals initiates early in urogenital tract development [46] and is followed by a second wave of apoptosis in and around the mesonephric tubules, specifically in the mid to caudal region of the mesonephros [46]. Gene expression studies in human kidney development indicate that caspase-3-mediated apoptosis is associated with regression of these transient kidneys (Fig. 1b) [47]. Interestingly, these studies also suggest that p53 may be the upstream inducer of death [47]; however, in transgenic mice, no defects in mesonephric tubule regression or metanephric kidney apoptosis have been noted [48]. Finally, clearance of the apoptotic bodies resulting from disintegration of the mesonephros appears to be mediated by hemopoietically derived specialist macrophages which aggregate within and adjacent to mesonephric tubules [49].
Aside from the upper Wolffian duct, we and others have recently demonstrated that the common nephric duct (CND) must also undergo programmed cell death to generate patent connections with the bladder [7, 8]. This process involves a gradient of apoptosis mediated by both caspase-3 and caspase-8 originating along the CND proximal to the bladder and decreasing toward the junction with the ureter (Fig. 1b) [7]. In this fashion, a controlled descent of the ureter to the bladder is possible, subsequently allowing the distal section of the ureter to lie along the bladder and also be eliminated by apoptosis [7, 8, 50]. Little is known about the induction of ductal cell death in this situation, although it is apparent that the signal(s) activate both the intrinsic and extrinsic pathways of apoptosis. It has been proposed that retinoic acid (RA) signaling emanating from the urogenital sinus may be this mediator (Fig. 1b), as knockout mice for the RA-synthesizing enzyme, retinaldehyde dehydrogenase-2 (Raldh2), lack CND apoptosis [8]. Furthermore, it has been suggested that the Ret receptor tyrosine kinase may potentiate RA signaling during later apoptotic elimination of the distal ureter (Fig. 1b) [8]. However, Ret has also been implicated in the earlier survival of the CND, as it has been demonstrated that it is down-regulated by receptor protein tyrosine phosphatase (RPTP)-σ, an inducer of CND cell death [7]; thus, a counterbalance of pro-survival and pro-death signaling in controlling the rate of ureter maturation is beginning to be elucidated. However, due to presumed redundancy among the LAR-family of RPTPs, namely, Ptprf and Ptprs, loss of both is required to reveal the pro-apoptotic role in the CND (Fig. 1b). Genetic ablation of Ptprf;Ptprs results in a persistence of the CND in over half of the mutants, accompanied by a specific decrease in caspase-3, but not caspase-8, activation, thus implicating the action of the LAR-family RPTPs in control of the mitochondrial pathway of apoptosis [7]. Intriguingly, it seems that induction of both branches of apoptosis is necessary for proper ureter maturation; a blockade in the intrinsic pathway impedes apoptotic degeneration of the CND which is not rescued by the continued presence of activated caspase-8. However, the observation of incomplete penetrance in Ptprf;Ptprs double mutants does suggest that alternative mechanisms activate a sufficient level of apoptosis to allow for ureter maturation. Whether this is achieved through compensation by an additional phosphatase or caspase signaling pathway has yet to be determined.
A similar requirement for the intrinsic apoptotic pathway is seen in the development of the metanephros. During normal ureteric bud invasion and branching in the mouse, apoptotic bodies are observed in both the epithelial and mesenchymal compartments, although the highest proportion of cell death occurs in the metanephric mesenchyme (Fig. 1b) [21, 44, 47]. Most apoptosis in the mesenchyme occurs close to, but not within differentiated nephron epithelia, presumably as it is preserved by pro-survival signals, although as time progresses more apoptotic bodies are seen within the podocyte region [21, 44, 47]. This pattern of apoptosis is postulated to facilitate reciprocal signaling between the branching ureteric bud and condensing metanephric mesenchyme by removing non-differentiating mesenchyme [21, 44]. In support of this suggestion, inhibition of caspase-3 and caspase-9, but not caspase-8, hinders branching morphogenesis and nephrogenesis in metanephric explants, and this is associated with the inhibition of apoptosis in non-diferentiated mesenchymal cells (Fig. 1b) [51, 52]. Interestingly, the blocking of caspase-3 is associated with a stronger inhibitory effect on metanephric development than the elimination of caspase-9 [51], suggesting the existence of other mechanisms to activate caspase-3 that are independent of the apoptosome. However, even in the absence of caspase-3 in mice, normal cell death occurs in metanephric development, highlighting again the redundancy in apoptosis [53].
Although the pattern and ultimate execution of apoptosis in the developing kidney have been described, many questions remain to be answered, especially regarding upstream of the apoptotic pathway itself. The most basic question of whether all the lineages that die in the kidney do so because it is intrinsic or induced is yet unknown, as are the regulators of this process. Furthermore, the strict control of programmed cell death and the multiple redundancies within the process suggest that apoptosis at a specific time in a subset of urogenital cells is highly important; however, the developmental purposes behind such cell death are sometimes unclear. Finally, and perhaps most challenging, the relationship between survival and death and the interplay between the molecules controlling each remain elusive.
Conclusion
Though many questions remain unanswered over the regulation and roles that survival and death play in the morphogenesis of the mammalian kidney, their importance to appropriate development is undeniable. Multiple abnormalities of the kidney and urinary tract have been described in relation to loss of either a pro-survival or pro-apoptotic gene, including kidney agenesis and dysplasia, and ureter hypoplasia [7, 8, 25–29, 31, 33, 54]. Congenital abnormalities of these types occur frequently in the pediatric population, often impacting negatively on the life of the individual. As such, there is an urgent need to identify loci associated with these phenotypes, and although many have been identified in recent years, a significant proportion of patients have no known mutations in these [55]. It is possible that the survival and apoptosis pathways reviewed herein, which have been demonstrated to play a crucial role in shaping the urogenital system, will provide new candidate loci to screen for defects in kidney and ureter development.
References
Bouchard M (2004) Transcriptional control of kidney development. Differentiation 72:295–306
Kobayashi A, Behringer RR (2003) Developmental genetics of the female reproductive tract in mammals. Nat Genet 4:969–980
Vainio S, Lin Y (2002) Coordinating early kidney development: lessons from gene targeting. Nat Genet 3:533–543
Costantini FK, Kopan R (2010) Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev Cell 18:698–712
Savill J (1995) Apoptosis: will cell death add life to nephrology? Nephrol Dial Transplant 10:1977–1979
Koseki C (1993) Cell death programmed in uninduced metanephric mesenchymal cells. Pediatr Nephrol 7:609–611
Uetani N, Bertozzi K, Chagnon MJ, Hendriks W, Tremblay ML, Bouchard M (2009) Maturation of ureter-bladder connection in mice is controlled by LAR family receptor protein tyrosine phosphatases. J Clin Invest 119:924–935
Batourina E, Tsai S, Lambert S, Sprenkle P, Viana R, Dutta S, Hensle T, Wang F, Niederreither K, McMahon AP, Carroll TJ, Mendelsohn CL (2005) Apoptosis induced by vitamin A signaling is crucial for connecting the ureters to the bladder. Nat Genet 37:1082–1089
Mendelsohn CL (2009) Using mouse models to understand normal and abnormal urogenital tract development. Organogenesis 5:306–314
Taylor RC, Cullen SP, Martin S (2008) Apoptosis: controlled demolition at the cellular level. Nat Mol Cell Biol 9:231–241
Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH (2006) Hierarchical regulation of mitochondrion-dependent apoptosis by Bcl-2 subfamilies. Nat Cell Biol 8:1348–1358
Merino D, Giam M, Hughes PD, Siggs OM, Heger K, O'Reilly LA, Adams JM, Strasser A, Lee E, Fairlie WD, Bouillet P (2009) The role of BH3-only protein Bim extends beyond inhibiting Bcl-2-like prosurvival proteins. J Cell Biol 186:355–362
Chipuk JE, Moldoveanu T, Liambi F, Parsons MJ, Green DR (2010) The Bcl-2 family reunion. Mol Cell 37:299–310
Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CY, Sasaki T, Elia AJ, Cheng HY, Ravagnan L, Ferri KF, Zamzami N, Wakeham A, Hakem R, Yoshida H, Kong YY, Mak TW, Zuniga-Pllucker JC, Kroemer G, Penninger JJ (2001) Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 410:549–554
Lakhani SA, Masud A, Kuida K, Porter GA, Booth CJ, Mehal WZ, Inayat I, Flavell RA (2006) Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science 311:847–851
Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C, Karasuyama H, Su MS, Rakic P, Flavell RA (1998) Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell 94:325–337
Schafer ZK, Kornbluth S (2006) The apoptosome: physiological, developmental, and pathological modes of regulation. Dev Cell 10:549–561
Peter ME, Krammer PH (2003) The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ 10:26–35
Billen LP, Shamas-Din A, Andrews DW (2008) Bid: a Bax-like BH3 protein. Oncogene 27[Suppl 1]:S93–S104
Koseki C, Herzlinger D, al-Awqati Q (1992) Apoptosis in metanephric development. J Cell Biol 119:1327–1333
Coles HS, Burne JF, Raff MC (1993) Large-scale normal cell death in the developing rat kidney and its reduction by epidermal growth factor. Development 118:777–784
Barasch J, Qiao J, McWilliams G, Chen D, Oliver JA, Herzlinger D (1997) Ureteric bud cells secrete multiple factors, including bFGF, which rescue renal progenitors from apoptosis. Am J Physiol 273:F757–F767
Perantoni AO, Dove LF, Karavanova I (1995) Basic fibroblast growth factor can mediate the early inductive events in renal development. Proc Natl Acad Sci USA 92:4696–4700
Zhou M, Sutliff RL, Paul RJ, Lorenz JN, Hoying JB, Haudenschild CC, Yin M, Coffin JD, Kong L, Kranias EG, Luo W, Boivin GP, Duffy JJ, Pawlowski SA, Doetschman T (1998) Fibroblast growth factor 2 control of vascular tone. Nat Med 4:201–207
Grieshammer U, Cebrian C, Ilagan R, Meyers E, Herzlinger D, Martin GR (2005) FGF8 is required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons. Development 132:3847–3857
Perantoni AO, Timofeeva O, Naillat F, Richman C, Pajni-Underwood S, Wilson C, Vainio S, Dove LF, Lewandoski M (2005) Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development 132:3859–3871
Poladia DP, Kish K, Kutay B, Hains D, Kegg H, Zhao H, Bates CM (2006) Role of fibroblast growth factor receptors 1 and 2 in the metanephric mesenchyme. Dev Biol 291:325–339
Miyazaki Y, Oshima K, Fogo A, Ichikawa I (2003) Evidence that bone morphogenetic protein 4 has multiple biological functions during kidney and urinary tract development. Kidney Int 63:835–844
Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G (1995) BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev 9:2808–2820
Dudley AT, Godin RE, Robertson EJ (1999) Interaction between FGF and BMP signaling pathways regulates development of metanephric mesenchyme. Genes Dev 13:1601–1613
Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R (1993) WT-1 is required for early kidney development. Cell 74:679–691
Donovan MJ, Natoli TA, Sainio K, Amstutz A, Jaenisch R, Sariola H, Kreidberg JA (1999) Initial differentiation of the metanephric mesenchyme is independent of WT1 and the ureteric bud. Dev Genet 24:252–262
Bouchard M, Souabni A, Mandler M, Neubuser A, Busslinger M (2002) Nephric lineage specification by Pax2 and Pax8. Genes Dev 16:2958–2970
Dziarmaga A, Clark P, Stayner C, Julien JP, Torban E, Goodyer P, Eccles M (2003) Ureteric bud apoptosis and renal hypoplasia in transgenic PAX2-Bax fetal mice mimics the renal-coloboma syndrome. J Am Soc Nephrol 14:2767–2774
Bouchard M, Pfeffer P, Busslinger M (2000) Functional equivalence of the transcription factors Pax2 and Pax5 in mouse development. Development 127:3703–3713
Narlis M, Grote D, Gaitan Y, Boualia SK, Bouchard M (2007) Pax2 and pax8 regulate branching morphogenesis and nephron differentiation in the developing kidney. J Am Soc Nephrol 18:1121–1129
Dziarmaga A, Eccles M, Goodyer P (2006) Suppression of ureteric bud apoptosis rescues nephron endowment and adult renal function in Pax2 mutant mice. J Am Soc Nephrol 17:1568–1575
Dziarmaga A, Hueber PA, Iglesias D, Hache N, Jeffs A, Gendron N, Mackenzie A, Eccles M, Goodyer P (2006) Neuronal apoptosis inhibitory protein is expressed in developing kidney and is regulated by PAX2. Am J Physiol Ren Physiol 291:F913–F920
Sorenson CM, Rogers SA, Korsmeyer SJ, Hammerman MR (1995) Fulminant metanephric apoptosis and abnormal kidney development in bcl-2-deficient mice. Am J Physiol 268:F73–F81
Nagata M, Nakauchi H, Nakayama K, Loh D, Watanabe T (1996) Apoptosis during an early stage of nephrogenesis induces renal hypoplasia in bcl-2-deficient mice. Am J Pathol 148:1601–1611
Bouillet P, Cory S, Zhang LC, Strasser A, Adams JM (2001) Degenerative disorders caused by Bcl-2 deficiency prevented by loss of its BH3-only antagonist Bim. Dev Cell 1:645-653.
Sorenson CM, Sheibani N (1999) Focal adhesion kinase, paxillin, and bcl-2: analysis of expression, phosphorylation, and association during morphogenesis. Dev Dyn 215:371–382
Sorenson CM (2004) Interaction of bcl-2 with Paxillin through its BH4 domain is important during ureteric bud branching. J Biol Chem 279:11368–11374
Foley JG, Bard JB (2002) Apoptosis in the cortex of the developing mouse kidney. J Anat 201:477–484
Yin Y, Lin C, Ma L (2006) MSX2 promotes vaginal epithelial differentiation and wolffian duct regression and dampens the vaginal response to diethylstilbestrol. Mol Endocrinol 20:1535–1546
Pole RJ, Qi BQ, Beasley SW (2002) Patterns of apoptosis during degeneration of the pronephros and mesonephros. J Urol 167:269–271
Carev D, Krnic D, Saraga M, Sapunar D, Saraga-Babic M (2006) Role of mitotic, pro-apoptotic and anti-apoptotic factors in human kidney development. Pediatr Nephrol 21:627–636
Saifudeen Z, Dipp S, Stefkova J, Yao X, Lookabaugh S, El-Dahr SS (2009) p53 regulates metanephric development. J Am Soc Nephrol 20:2328–2337
Camp V, Martin P (1996) The role of macrophages in clearing programmed cell death in the developing kidney. Anat Embryol Berl 194:341–348
Uetani N, Bouchard M (2009) Plumbing in the embryo: developmental defects of the urinary tracts. Clin Genet 75:307-317.
Araki T, Hayashi M, Nakanishi K, Morishima N, Saruta T (2003) Caspase-9 takes part in programmed cell death in developing mouse kidney. Nephron Exp Nephrol 93:e117–e124
Araki T, Saruta T, Okano H, Miura M (1999) Caspase activity is required for nephrogenesis in the developing mouse metanephros. Exp Cell Res 248:423–429
Kuida K, Zheng TS, Na S, Kuan C, Yang D, Karasuyama H, Rakic P, Flavell RA (1996) Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature 384:368–372
Porteous S, Torban E, Cho NP, Cunliffe H, Chua L, McNoe L, Ward T, Souza C, Gus P, Giugliani R, Sato T, Yun K, Favor J, Sicotte M, Goodyer P, Eccles M (2000) Primary renal hypoplasia in humans and mice with PAX2 mutations: evidence of increased apoptosis in fetal kidneys of Pax2(1Neu) +/- mutant mice. Hum Mol Genet 9:1–11
Kerecuk L, Schreuder MF, Woolf AS (2008) Renal tract malformations: perspectives for nephrologists. Nat Clin Pract Nephrol 4:312–325
Acknowledgements
We wish to thank Sami Kamel Boualia and Alana Nguyen for critical reading of the manuscript. Work in the Bouchard lab is supported by an operating grant from the Canadian Institute for Health Research (MOP-84343). M. Bouchard holds a Canada Research Chair in Developmental Genetics of the Urogenital System. K. Stewart is supported by funding from Fonds de la Recherche en Santé du Quebec.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stewart, K., Bouchard, M. Kidney and urinary tract development: an apoptotic balancing act. Pediatr Nephrol 26, 1419–1425 (2011). https://doi.org/10.1007/s00467-011-1788-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-011-1788-y