Abstract
Semaphorins are guidance proteins that play important roles in organogenesis and disease. Expression of class 3 semaphorins and their receptors is regulated during kidney development. Gain- and loss-of-function experiments demonstrated that tight semaphorin3a gene dosage is required for podocyte differentiation, and for the establishment of a normal glomerular filtration barrier. Sema3a modulates kidney vascular patterning acting as a negative regulator of endothelial cell migration and survival. Excess podocyte semaphorin3a expression causes glomerular disease in mice. In addition, Sema3a is a negative regulator of ureteric bud branching, whereas Sema3c is a positive regulator of ureteric bud and endothelial cell branching morphogenesis. In summary, secreted semaphorins modulate ureteric bud branching, vascular patterning, and podocyte-endothelial crosstalk, suggesting that they play a role in renal disease. Understanding the signaling pathways downstream from semaphorin receptors will provide insight into the mechanism of action of semaphorins in renal pathology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Semaphorins are guidance proteins that influence cellular morphology and function, and play important roles in organogenesis and disease [1]. Semaphorins, initially identified as axon repellents and subsequently recognized as attractant and repellent cues for multiple cell types, are phylogenetically conserved from C. Elegans to humans. Semaphorin proteins are defined by a common 500-amino-acid sema domain, a plexin-semaphorin-integrin (PSI) domain, and a specific C-terminal domain, which allows to group them in classes 1–8. Semaphorins are membrane bound (class 1, 4-6), secreted (class 2-3) or glycosyl phosphatidyl-inositol linked (class 7). The diverse protein structure and binding affinity to multiple receptor complexes provides the basis for semaphorin long or short-acting guidance cues. Class 3 semaphorins include sema3a through sema3g [2]. The receptor complex that transduces class 3 semaphorin signals involves several proteins. Neuropilins 1 and 2 are semaphorin-binding receptors that form a complex with the signaling receptors plexins A and D [3, 4], except for sema3e, which binds plexinD1 directly. Neuropilin1 and 2 exhibit binding specificities for class 3 semaphorins: sema3a, sema3b, and sema3c bind to neuropilin1, whereas only Sema3b, 3c, and 3f bind neuropilin2 [5, 6]. Neuropilins are also co-receptors for VEGF-A [1]. Neuropilins are single transmembrane glycoproteins that share about 40% sequence identity and consist of a large extracellular domain, and very short transmembrane and cytoplasmic domains [3, 7]. Neuropilin extracellular A and B domains encode binding sites for sema3a and VEGF165, which are distinct but adjacent, and have binding enhancer sequences in each other’s binding regions [7], explaining some of the observed functional competition between sema3a and VEGF165 [8–10]. Plexins are conserved transmembrane proteins that transduce semaphorin signals [4, 11]. Sema3a binds neuropilin1 to assemble and activate a neuropilin-plexin holoreceptor complex [4]. In addition, secreted semaphorin signaling complexes can include cell adhesion molecules, such as L1 [12], receptor tyrosine kinase (RTK) co-receptors, such as VEGFR2 and OTK [13], and integrin receptors [14]. Therefore, cell-specific expression of semaphorin receptors and diverse co-receptors dictate the guidance cue effects on cell morphology or function. The intracellular signaling downstream from plexinA involves multiple pathways, including protein kinases such as PI3K/Akt, Fyn, and GSK3β, which interact with CRMPs and thereby regulate microtubule and actin dynamics, as well as Rho GTPases and MICAL, which also regulate actin dynamics (reviewed in [1, 15]). This review is focused on the role of class 3 semaphorins, specifically sema3a and sema3c, in kidney development and disease.
Expression of class 3 semaphorins and their receptors is regulated during kidney development
Sema3a and sema3f mRNA expression are detected in E12.5 mouse and E14 rat kidneys, and are developmentally regulated, such that expression levels are higher in E14 and newborn than in adult rat kidneys, and the localization of transcripts becomes limited to fewer structures as maturation proceeds [16]. In developing kidneys, sema3a and sema3f transcripts localize to S-shape bodies and ureteric buds. In mature kidneys, sema3a and sema3f localize to podocytes, distal tubules and collecting ducts. We have also identified Sema3b,3c,3d,3e and 3g mRNA expression in newborn mouse kidneys [17]. Sema3a and sema3f receptors, neuropilin-1 and neuropilin-2, are also expressed at higher levels in developing kidneys as compared to adult ones, and localize to the vascular cleft of S-shaped bodies. PlexinA1 mRNA is expressed at low levels in the kidney at E14, whereas plexinB1 and plexinB2 mRNAs are expressed in glomeruli and cap mesenchyme at E16-E18 [18]. Class 3 semas (a-f), neuropilin 1 and 2, and plexinsA1-A3 and D1 are expressed in cultured podocytes [19]. The fact that Sema3a and 3f have overlapping expression patterns suggests that semaphorins may work in concert in the kidney. Congruent with this, Sema3b and sema3e mRNAs were upregulated in newborn kidneys harboring increased Sema3a expression or Sema3a deletion, respectively [17]. Notably, Sema3a and sema3f transcripts localized to sites of vascular endothelial growth factor (VEGF-A) expression, and semaphorin receptors neuropilins 1 and 2 localized to sites of VEGF receptor-2 (VEGFR2) expression, i.e., podocytes, endothelial cells, and collecting tubules [9, 16, 20, 21]. As VEGF164 and Sema3a compete for neuropilin1 binding [7, 8, 22], we proposed that the signaling pathways might interact and modulate renal development.
Sema3a modulates kidney vascular patterning acting as a negative regulator of endothelial cell migration and survival
To investigate the role of Sema3a in kidney vascular patterning, gain- and loss-of-function mouse models were used [17]. Sema3a null kidneys exhibited abnormal vascular patterning, with increased Flk1-LacZ positive endothelial cells throughout the kidney, and increased endothelial cells filling capillary lumens, suggesting that the absence of Sema3a-mediated inhibition of endothelial migration led to abnormal vessel patterning and excess endothelial cells. We determined that the increased endothelial cell number in Sema3a-/- mutants was due to decreased endothelial cell apoptosis. Conversely, mice overexpressing Sema3a in podocytes during renal organogenesis developed endothelial cell apoptosis and glomerular hypoplasia. In addition, recombinant Sema3a inhibited endothelial cell migration into embryonic kidney explants in co-culture experiments and in migration assays, demonstrating that sema3a functions as a chemo-repellent guidance cue for glomerular endothelial cell migration. These findings are consistent with the Sema3a mutant glomerular phenotype and with previous reports on endothelial cells [8]. By contrast, VEGF-A promotes endothelial cell survival and acts as a chemoattractant, driving endothelial cell migration into the vascular cleft of the S-shaped nephron [20, 23]. Collectively, these findings suggest that a balance between VEGF-A and Sema3a signaling pathways regulates endothelial cell number and vascular patterning during glomerulogenesis.
Sema3a is a negative regulator of ureteric bud branching
The role of Sema3a in the developing ureteric bud was examined in vitro using embryonic kidney explants exposed to recombinant Sema3a or Sema3a antisense morpholino and in vivo in Sema3a-/-:Hoxb7GFP mice [24]. Hoxb7GFP mice express GFP in ureteric bud lineage cells [25]. Explant exposure to Sema3a reduced ureteric bud branching, whereas Sema3a knockdown and Sema3a gene deletion increased bud branching, indicating that Sema3a is a negative regulator of ureteric bud branching morphogenesis. Moreover, explant exposure to recombinant Sema3a decreased GDNF expression and Ret phosphorylation. Neuropilin-1, the binding sema3a receptor, was identified in ureteric bud derived cells. VEGF-A stimulates proliferation and branching of ureteric bud via phosphorylation of the Ret receptor and upregulation of its major ligand, glial-derived neurotrophic factor (GDNF) [9, 26]. Sema3a-induced inhibition of ureteric bud branching was rescued by VEGF165, suggesting that the balance between Sema3a and VEGF signaling pathways regulates ureteric bud branching and thereby modulates nephron number [24]. Together, Sema3a and VEGF signaling pathways coordinate vascular and epithelial patterning during kidney morphogenesis, via direct effects on vasculogenesis, angiogenesis and ureteric bud branching [17, 24]. Two class 4 semaphorins, sema4c and sema4d, have been recently shown to be negative modulators of ureteric bud branching, suggesting overlapping functions to sema3a [27, 28].
The role of Sema3a in podocyte-endothelial crosstalk
Sema3a expression in the mature podocyte persists after completion of glomerular development [16]. In vitro, recombinant Sema3a induced podocyte apoptosis and decreased the association of the slit-diaphragm proteins nephrin, podocin and CD2AP, suggesting that podocyte Sema3a has cell autonomous functions [19]. In vivo, overexpression of podocyte Sema3a during kidney organogenesis resulted in a delay of foot process development and absence of slit diaphragms [17]. This was associated with downregulation of WT-1 and nephrin, suggesting that Sema3a signaling modulates podocyte differentiation signaling pathways. Endothelial apoptosis induced by excess podocyte Sema3a, and excess endothelial cells in Sema3a-/-, strongly suggested that Sema3a has non-cell autonomous functions in glomerular development, involving podocyte and endothelial cell crosstalk [17].
To investigate the role of Sema3a in the mature kidney, recombinant Sema3a was given intraperitoneally to wild-type mice [10], and Sema3a overexpression was induced in adult mice. Sema3a administration caused acute proteinuria, foot process effacement, and endothelial cell swelling, which were reversible within 24 h and were abrogated by co-administration of rVEGF165 [10]. Similarly, overexpression of podocyte Sema3a in adult mice induced glomerular disease (Reidy et al. 2011 unpublished data used with permission). Sema3a-induced abnormalities were associated with downregulation of VEGFR2, which was also detected in mice overexpressing podocyte Sema3a during development [17], and isolated podocytes exposed to sema3a [19], suggesting that excess sema3a signaling interferes with VEGF-A signaling. Consistent with this, Sema3a overexpression glomerular phenotype resembles podocyte VEGF knockdown during organogenesis (Veron et al. 2011 unpublished results used with permission). Overexpression of VEGF164 in podocytes induces distinct glomerular diseases, depending on the developmental stage and magnitude of VEGF excess [21, 29, 30]. Collectively, these findings suggest that the balance of Sema3a and VEGF-A expression in podocytes is important not only during kidney development but also to maintain the integrity of the glomerular filtration barrier in the adult kidney. Ongoing studies are exploring how Sema3a and VEGF-A signals interact with the slit-diaphragm signaling complex.
Sema3a expression is increased in experimental models of proteinuric kidney disease
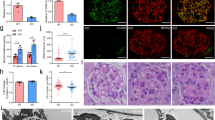
We have identified increased Sema3a mRNA and protein expression in experimental models of glomerular disease, including db/db kidneys and PAN nephrosis (Fig. 1), as well as streptozotocin-induced diabetes [31]. Interestingly, sema3a expression was decreased in HIV-infected podocytes, while HIV-induced collapsing focal glomerulosclerosis was associated with upregulation of VEGF, neuropilin-1, VEGFR1, and VEGFR2 [32]. These findings further support the concept that the balance between Sema3a and VEGF-A signaling continues to be required in podocytes after completion of kidney development, and suggests that guidance proteins and angiogenic factors, such as Sema3a and VEGF, play a pathogenic role in human proteinuric kidney disease.
Sema3a overexpression in glomerular disease: a qPCR: sema3a mRNA increased ∼4-fold in PAN nephrotic rat glomeruli; b sema3a mRNA increases 2-fold in Db/Db diabetic glomeruli. All values were calculated as described [18] and normalized to GAPDH
Sema3c is a positive regulator of ureteric bud and endothelial cell branching morphogenesis
The role of Sema3c in the developing ureteric bud was examined in vitro using embryonic kidney explants exposed to recombinant Sema3c, and in vivo in Sema3c-/-:Hoxb7GFP mice. Recombinant sema3c induced an increase in ureteric bud branching (Fig. 2a, b), whereas Sema3c null mutants showed decreased ureteric bud branching (Fig. 2c, d). Interestingly, Sema3a/3c double null mutant mice (Sema3a-/-:Sema3c-/-:Hoxb7GFP) revealed a more severe branching phenotype, as compared to the wild-type or any heterozygote combination (Fig. 2e-f). These findings suggest that Sema3a and Sema3c modulate ureteric bud branching in opposite directions, but Sema3c plays a more robust role, such that deletion of both genes results in decreased ureteric bud branching. Moreover, the litter size from double heterozygote crossbreeding decreased from 10.2 ± 0.60 at E13.5 to 6.3 ± 1.2 at E.14.5 (p < 0.05), indicating embryonic lethality. Further, at E15.5 no double null embryo was alive (n = 0/8), likely due to complex cardiac abnormalities (data not shown). By contrast, double heterozygotes appeared normal, and both Sema3a-/- and Sema3c-/- were perinatal lethal, due to concentric cardiomyopathy and severe outflow tract abnormalities, respectively, as reported [33, 34].
Sema3c stimulates ureteric bud branching: a–b paired metanephros cultured for 4 days, FITC-Dolichos biflora agglutinin labeled, a control, b sema3c-treated (250 ng/ml) showing increased size and ureteric bud branching; c–d Ex vivo dissected E12.5 Sema3c+/+:Hoxb7GFP and Sema3c-/-:Hoxb7GFP kidneys from littermates showing decreased branching in the mutant; e–f Ex vivo dissected Sema3a+/+:Sema3c+/+:Hoxb7GFP (wild-type) and Sema3a-/-:Sema3c-/-:Hoxb7GFP (double mutant) littermate kidneys, showing dysmorphic ureteric buds and decreased branching in the double mutant
Sema3c plays an important role in endothelial cell guidance during vascular development, as evidenced by cardiac outflow tract patterning abnormalities in null mutant mice [34]. We examined the mechanisms whereby Sema3c regulates endothelial function [35]. Sema3c promotes glomerular endothelial proliferation and survival, enhances cell adhesion to fibronectin and collagen I, and stimulates β1-integrin activity [35]. In addition, Sema3c induces directional cell migration [35] and increases endothelial tube and network formation (Fig. 3). These effects are mediated by Sema3c-induced VEGF120 secretion, via neuropilin-1 and neuropilin-2 signaling, and are independent of integrin and VEGFR2 signals [35]. Collectively, these findings imply a new paradigm involving a crosstalk between sema3c and VEGF-A signaling resulting in similar functions, and contributing to the complexity of antagonistic sema3a vs. sema3c signals.
Sema3c and VEGF165 stimulate endothelial cell tube formation and sema3a inhibits tube formation. Mouse glomerular endothelial cells plated on collagen I gels, serum free, for 48 h and labeled with Cell Tracker®: a Control endothelial cell network; b sema3c (300 ng/ml) induces extensive capillary-like network formation; c VEGF165 (30 ng/ml) induces capillary-like network formation; d sema3a (250 ng/ml) abrogates most of endothelial cell tube formation
Conclusions and outlook
Semaphorins play an important role in patterning the developing kidney by modulating ureteric bud branching, vascularization, podocyte differentiation, and establishment of the glomerular filtration barrier. Semaphorin signals also contribute to maintain the integrity of the glomerular filtration barrier in the adult kidney. While sema3a dysregulation causes proteinuric renal disease in mice, there is still a gap to fill on the mechanism of action of semaphorins in renal pathology. Understanding the signaling pathways downstream from semaphorin receptors in renal cells will provide insight into how they regulate cell function and interactions. Defining the cross talk between semaphorin signaling and other pathways, including growth factors and integrins, will enable to identify a molecular hierarchy towards using the semaphorin cascade as novel therapeutic target.
References
Tran TS, Kolodkin AL, Bharadwaj R (2007) Semaphorin regulation of cellular morphology. Annu Rev Cell Dev Biol 23:263–292
Semaphorin Nomenclature Committee (1999) Unified nomenclature for the semaphorins/collapsins. Cell 97(5):551–552
Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD (1997) Neuropilin is a semaphorin III receptor. Cell 90:753–762
Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, Fujisawa H, Strittmatter SM (1999) Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell 99:59–69
Chen H, He Z, Bagri A, Tessier-Lavigne M (1998) Semaphorin-neuropilin interactions underlying sympathetic axon responses to class III semaphorins. Neuron 21:1283–1290
Chen H, Chedotal A, He Z, Goodman CS, Tessier-Lavigne M (1997) Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron 19:547–559
Geretti E, Shimizu A, Klagsbrun M (2008) Neuropilin structure governs VEGF and semaphorin binding and regulates angiogenesis. Angiogenesis 11:31–39
Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M (1999) Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol 146:233–242
Tufro A, Teichman J, Banu N, Villegas G (2007) Crosstalk between VEGF-A/VEGFR2 and GDNF/RET signaling pathways. Biochem Biophys Res Commun 358:410–416
Tapia R, Guan F, Gershin I, Teichman J, Villegas G, Tufro A (2008) Semaphorin3a disrupts podocyte foot processes causing acute proteinuria. Kidney Int 73:7337–7340
Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, Chedotal A, Winberg ML, Goodman CS, Poo M, Tessier-Lavigne M, Comoglio PM (1999) Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 99:71–80
Castellani V, Chédotal A, Schachner M, Faivre-Sarrailh C, Rougon G (2000) Analysis of the L1-deficient mouse phenotype reveals cross-talk between Sema3A and L1 signaling pathways in axonal guidance. Neuron 27:237–249
Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Suto F, Kamei J, Aoki K, Yabuki M, Hori M, Fujisawa H, Kikutani H (2004) Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev 18:435–447
Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L, Logan M, Tessier-Lavigne M, Taniguchi M, Püschel AW, Bussolino F (2003) Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature 424:391–397
Roth L, Koncina E, Satkauskas S, Crémel G, Aunis D, Bagnard D (2009) The many faces of semaphorins: from development to pathology. Cell Mol Life Sci 66:649–666
Villegas G, Tufro A (2002) Ontogeny of semaphorins 3A and 3F and their receptors neuropilins 1 and 2 in the kidney. Gene Expr Patterns 2(1–2):151–155
Reidy KJ, Villegas G, Teichman J, Veron D, Shen W, Jimenez J, Thomas D, Tufro A (2009) Semaphorin3a regulates endothelial cell number and podocyte differentiation during glomerular development. Development 136:3979–3989
Perälä NM, Immonen T, Sariola H (2005) The expression of plexins during mouse embryogenesis. Gene Expr Patterns 5:355–362
Guan F, Villegas G, Teichman J, Mundel P, Tufro A (2006) Autocrine class 3 semaphorin system regulates slit diaphragm proteins and podocyte survival. Kidney Int 69:1564–1569
Tufro A, Norwood VF, Carey RM, Gomez RA (1999) Vascular endothelial growth factor induces nephrogenesis and vasculogenesis. J Am Soc Nephrol 10:2125–2134
Veron D, Reidy KJ, Bertuccio C, Teichman J, Villegas G, Jimenez J, Shen W, Kopp JB, Thomas DB, Tufro A (2010) Overexpression of VEGF-A in podocytes of adult mice causes glomerular disease. Kidney Int 77:989–999
Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M (1998) Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 927:35–745
Tufro A (2000) VEGF spatially directs angiogenesis during metanephric development in vitro. Dev Biol 227:558–566
Tufro A, Teichman J, Woda C, Villegas G (2008) Semaphorin3a inhibits ureteric bud branching morphogenesis. Mech Dev 125:558–568
Srinivas S, Goldberg MR, Watanabe T, D’Agati V, al-Awqati Q, Costantini F (1999) Expression of green fluorescent protein in the ureteric bud of transgenic mice: a new tool for the analysis of ureteric bud morphogenesis. Dev Genet 24(3–4):241–251
Karihaloo A, Karumanchi SA, Cantley WL, Venkatesha S, Cantley LG, Kale S (2005) Vascular endothelial growth factor induces branching morphogenesis/tubulogenesis in renal epithelial cells in a neuropilin-dependent fashion. Mol Cell Biol 25:7441–7448
Korostylev A, Worzfeld T, Deng S, Friedel RH, Swiercz JM, Vodrazka P, Maier V, Hirschberg A, Ohoka Y, Inagaki S, Offermanns S, Kuner R (2008) A functional role for semaphorin 4D/plexin B1 interactions in epithelial branching morphogenesis during organogenesis. Development 135:3333–3343
Perälä N, Jakobson M, Ola R, Fazzari P, Penachioni JY, Nymark M, Tanninen T, Immonen T, Tamagnone L, Sariola H (2010) Sema4C-Plexin B2 signalling modulates ureteric branching in developing kidney. Differentiation. doi:https://doi.org/10.1016/j.diff.2010.10.001
Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE (2003) Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 111:707–716
Veron D, Reidy K, Marlier A, Bertuccio C, Villegas G, Jimenez J, Kashgarian M, Tufro A (2010) Induction of podocyte VEGF164 overexpression at different stages of development causes congenital nephrosis or steroid-resistant nephrotic syndrome. Am J Pathol 177:2225–2233
Veron D, Bertuccio CA, Marlier A, Reidy K, Garcia AM, Jimenez J, Velazquez H, Kashgarian M, Moeckel GW, Tufro A (2010) Podocyte VEGF164 overexpression causes severe nodular glomerulosclerosis in type 1 diabetic mice. Diabetologia. doi:https://doi.org/10.1007/s00125-010-2034-z
Korgaonkar SN, Feng X, Ross MD, Lu TC, D’Agati V, Iyengar R, Klotman PE, He JC (2008) HIV-1 upregulates VEGF in podocytes. J Am Soc Nephrol 19:877–883
Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC (1996) Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature 383:525–528
Feiner L, Webber AL, Brown CB, Lu MM, Jia L, Feinstein P, Mombaerts P, Epstein JA, Raper JA (2001) Targeted disruption of semaphorin 3C leads to persistent truncus arteriosus and aortic arch interruption. Development 128:3061–3070
Banu N, Teichman J, Dunlap-Brown M, Villegas G, Tufro A (2006) Semaphorin 3C regulates endothelial cell function by increasing integrin activity. FASEB J 20:2150–2152
Acknowledgements
This study was supported by NIH RO1-DK64187 and DK59333 (A.T.), K.R. was supported by NIH training grant T32 DK-007110. We thank K. Susztak (Albert Einstein College of Medicine) for providing the RNA samples from PAN rats and Db/Db mice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reidy, K., Tufro, A. Semaphorins in kidney development and disease: modulators of ureteric bud branching, vascular morphogenesis, and podocyte-endothelial crosstalk. Pediatr Nephrol 26, 1407–1412 (2011). https://doi.org/10.1007/s00467-011-1769-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-011-1769-1