Abstract
Bisphosphonates have been shown to attenuate ectopic calcification in experimental uremia. While they are known to reduce bone turnover, the effects on endochondral bone formation have not yet been addressed. To address this issue, we administered male Sprague-Dawley rats weekly subcutaneous injections of either vehicle or ibandronate (1.25 μg/kg body weight) for a total of 10 weeks. The rats were randomly allocated into one of four groups: (1) vehicle-treated, sham-operated rats; (2) ibandronate-treated, sham-operated rats; (3) vehicle-treated, 5/6 nephrectomized rats; (4) ibandronate-treated, 5/6 nephrectomized rats. Bones were double labeled with tetracycline and demeclocycline in vivo, and tibiae were removed for analysis. Weight gain was similar in all groups. Ibandronate reduced body length gain and tibial growth rate in the sham-operated animals but not in the rats showing chronic renal failure (CRF). The height of the proliferative zone of the epiphyseal growth plate was reduced in the ibandronate-treated controls and tended to be reduced in CRF rats. A significant correlation between tibial growth rate and height of the proliferative zone was observed. Mineral apposition rates were significantly reduced in ibandronate-treated, sham-operated rats and tended to be reduced in CRF rats. In conclusion, ibandronate interferes with tibial growth and bone mineralization in young rats with normal and reduced renal function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In patients suffering from chronic kidney disease, impaired mineral metabolism is strongly associated with both renal osteodystrophy and ectopic vascular calcification. The term chronic kidney disease-mineral bone disorder (CKD-MBD) was coined recently to highlight this association [1, 2]. The metabolism of calcium and phosphorous is under the tight control of three interwired endocrine circuits regulated by fibroblast-growth factor-23, vitamin D, and parathormone (PTH), while the skeleton serves as buffer and repository [3, 4]. Whereas this system under normal conditions is sufficiently stable and tolerant with respect to an acute excess or demand of calcium and/or phosphorus, it is directly affected by a declining kidney function. It has recently been shown that the skeleton and the vasculature are already affected in the early phase of CKD-MBD [5]. In addition, the prevalence of osteoporosis is rather high in CKD patients and even in the general population a strong association between atherosclerosis and osteoporosis has been shown [6].
Bisphosphonates are non-hydrolyzable pyrophosphate analogs that are able to interfere with osteoclast activation and bone resorption. Consequently, these compounds are widely prescribed in adults for the treatment of osteoporosis, bone metastases, and other conditions of osteoclast-mediated bone loss [7, 8]. Non-nitrogen containing bisphosphonates, such as etidronate, compete with ATP. By contrast, nitrogen-containing compounds, such as pamidronate and ibandronate, inhibit farnesyl pyrophosphate synthase, which is essential for posttranslational modification of small GTP-binding proteins [9]. Beyond this, individual bisphosphonates of either class differ with respect to the biological half-life, route of administration, side effects, and relative potencies to inhibit bone resorption and formation [7]. In this context it is worth mentioning that a recent study in experimental chronic uremia revealed that the etidronate dosage required to prevent arterial calcification also impaired bone mineralization [10]. In a more recent animal study, etidronate and pamidronate were shown to attenuate ectopic calcification and to reduce bone formation while leaving bone resorption virtually unaffected [11, 12]. Likewise, ibandronate has been shown to prevent arterial calcification in experimental uremia at dosages sufficient to prevent bone resorption, although the latter effect was assessed from previous experiments and not proven in the same animals [13–16]. In two other studies, ibandronate prevented the increase in erosion depth and bone turnover in nephrectomized rats, and olpadronate was able to lessen the decrease in bone mineral density (BMD) associated with high-turnover bone disease in uremic animals [17, 18].
Based on these results, it is likely that bisphosphonates interfere with endochondral ossification and thus longitudinal growth. This is of special importance in pediatric CKD patients since these patients are already prone to growth retardation. In the study reported here, we investigated the long-term effects of ibandronate on longitudinal growth and bone mineralization in young growing rats with normal and reduced renal function.
Materials and methods
Animals and experimental protocol
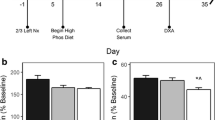
All animal handling and experiments outlined in this study were in accordance with the accepted principles of welfare of animals used in science and were approved by the regulatory authority. The time schedule and design of the experiment is given in Fig. 1. Male Sprague Dawley (Crl:CD) rats (n = 41) weighing 60–80 g were purchased from Charles River Laboratories, Sulzfeld, Germany and housed pair-wise at constant room temperature under a 12/12-h (light/dark) cycle. Rats underwent either 5/6 nephrectomy (n = 22) to induce chronic renal failure (CRF) or sham surgery (n = 19) under ketamine/xylanzine anesthesia (ketamine 10%; Essex Pharma, Munich, Germany; Rompune 2%, Bayer Vital, Leverkusen, Germany) essentially as described [19]. During post-operative recovery (3 days), the animals received metamizol via the drinking water. Animals were randomly allocated to receive weekly subcutaneous injections of either vehicle (0.9% saline) or ibandronate (Bondronate; Roche Diagnostics, Mannheim, Germany; 1.25 μg/kg body weight) for a total of 10 weeks. This dosage was selected since it was shown to be well tolerated in uremic rats and can be given once weekly [17, 20, 21]. Four groups were included in the study: (1) vehicle-treated, sham-operated rats; (2) ibandronate-treated, sham-operated rats; (3) vehicle-treated CRF rats; (4) ibandronate-treated CRF rats. Individual drug dosages were adjusted to the most recently recorded body weight. Animals received a commercial diet (Ssniff Spezialdiäten, Soest, Germany) containing 0.75% phosphorus, 1.01% calcium, 19% crude protein, and 1,000 IU/kg 25-hydroxy-cholecalciferol. All animals had access to the same amount of food as determined from the spontaneous food intake of the CRF rats receiving vehicle (pair-feeding), i.e. approximately 20 g per day. All animals had free access to drinking water throughout the study.
Rats were weighed twice weekly, the dietary intake was monitored daily, and blood was drawn (tail vein) at three time points (prior to treatment onset, during week 5, and at the end of the treatment period) to monitor serum creatinine, calcium, and phosphate. Blood pressure was measured by the tail-cuff method using a sphygmomanometer (TSE Systems, Bad Homburg, Germany) after 5 weeks of treatment [19]. For the assessment of longitudinal growth and mineral apposition rates, rats received tetracycline (30 mg/kg body weight) and demeclocycline (25 mg/kg body weight) 7 and 3 days, respectively, before sacrifice [22]. For the collection of urine, rats were housed in metabolic cages for 24 h before the end of the experiment. Animals were sacrificed by exsanguination through the abdominal aorta after ketamine/xylazine anesthesia 7 days after receiving the last treatment dosages. Tibiae were removed, dissected free of soft tissue, and transferred to 70% ethanol until further processing.
Methods
Blood and urine chemistry
Serum creatinine, calcium, and phosphate and urinary creatinine levels were determined with kinetic color tests (Diasys Diagnostic Systems/Greiner Biochemica, Flacht, Germany). The tests were performed manually and adapted to microtiter plate format; all samples were assayed in duplicate. Urinary 24-h creatinine clearances were calculated using the standard formula. PTH serum levels were measured by an immuno-assay specific for rat intact PTH (Immutopics, San Clemente, CA).
Histomorphometric analysis
The tibiae were dissected parallel to the sagittal plane, dehydrated in an ascending series of ethanol, and embedded in a glycol methacrylate resin (Technovit 7200 VLC; Heraeus Kulzer, Wehrheim, Germany) according to the technique of Donath and Breuner [23]. After polymerization, the tibiae were sawed into approximately 200-μm specimens parallel to the long axis and ground down to approximately 20 μm.
The unstained slides were investigated using an inverted fluorescence microscope (Leica DMI 4000) equipped with digital cameras [DFC 320 R2 (color) and DFC 350 Fx (black & white); Leica, Wetzlar, Germany] and corresponding software for data acquisition (Leica Application Suite, LAF6000). Images (100× magnification) were taken, and the interlabel distance was determined to calculate daily cortical mineral apposition rates and longitudinal growth, respectively [24, 25]. To assess the mean daily cortical mineral apposition rates, images were taken at the cortical periphery distal from the growth plate, and at least 60 measurements were performed per sample. Calculation of mean longitudinal growth per tibia is based on at least 80 measurements derived from three randomly selected images of the entire growth plate [24, 25].
The same sections were then etched with 1% formic acid (30 s) and stained with toluidine blue (0.7% in 0.1 M NaH2PO4/Na2HPO4 pH 7.2), as recently described [26]. Slides were viewed at a magnification of 100×, and images of the entire growth plate were taken to determine heights of the hypertrophic and proliferative zone. Established morphological criteria were applied to discriminate between hypertrophic and proliferative zones [27, 28]. At least 30 measurements per sample were taken. As the resting zone was almost absent in the vast majority of animals, the sum of the heights from the hypertrophic and proliferative zone was taken as the total height of the growth plate.
Statistical analysis
Data were analyzed using SPSS statistical package 15.0 (SPSS. Chicago, IL). Descriptive statistics, including the mean and standard deviation (SD), for continuous variables were computed. The Kolmogorov–Smirnov test was applied to assess normal distribution. Because hypotheses of normality were not rejected in any case, differences in continuous variables between the four groups were investigated using one-way analysis of variance (ANOVA) with Fisher's least significant difference (LSD) post-hoc tests. All p values resulted from two-sided statistical tests, and p < 0.05 was considered to be significant.
Results
Throughout the experiment, renal function was reduced by approximately 50% in CRF rats compared to controls, irrespective of ibandronate treatment (Table 1). Ibandronate-treated CRF rats experienced significantly elevated PTH serum levels compared to vehicle- or ibandronate-treated controls (each p < 0.05). In contrast, serum calcium and phosphate levels as well as mean blood pressure were quite comparable between groups (Table 1 and data not shown). Cumulative weight gain and daily food consumption (approx. 20 g per rat and day) were similar in all groups (Table 2 and data not shown). Ibandronate significantly reduced body length gain and tibial growth rate in sham-operated animals but not in CRF rats (Table 2, Fig. 2a). Accordingly, the height of the proliferative zone of the epiphyseal growth plate was significantly reduced in ibandronate-treated, sham-operated animals and tended to be reduced in CRF rats compared to the respective control animals (Table 2). Regardless of treatment and renal function, tibial growth rate and height of the proliferative zone of the growth plate were significantly correlated (Fig. 3). Total height of the growth plate and height of the hypertrophic zone were similar in all groups. In general, the mineral apposition rate was reduced by ibandronate, although this did not reach statistical significance in CRF rats (Fig. 2b).
Tibial growth rates (a) and mineral apposition rates (b) in sham-operated rats and 5/6 nephrectomized (5/6 SNx) rats after 10 weeks of treatment with weekly subcutaneous injections of vehicle (0.9% saline) or ibandronate (1.25 μg/kg body weight). Asterisk Significant differences between groups (p < 0.05)
Tibial growth rate as a function of the height of the proliferative zone of the growth plate. Data for vehicle (filled circle) and ibandronate (open circle) treated rats are given. A linear relationship between tibial growth rate and height of the proliferative zone is observed (r = 0.671, p < 0.001, Equation: tibial growth rate = 0.23 × height of the proliferative zone + 0.71)
Discussion
Pediatric and adult CKD patients are prone to develop ectopic calcifications and envisage a tremendously increased risk for cardiovascular morbidity and mortality [29–31]. Furthermore, vascular calcifications and impaired bone mineralization are significantly associated, not only in CKD patients but also in the general population [1, 6, 32]. While there is still no possibility to resolve firmly established vascular calcifications, bisphosphonates have emerged as a powerful tool for the treatment of osteoporosis and other conditions associated with increased osteoclast-mediated bone resorption [9]. The structure of this type of drugs closely resembles that of pyrophosphates, i.e. two phosphonate groups bound to a -C(OH)R moiety, with R representing the organic side chain. Both phosphonate groups together with the OH-group are responsible for the tight adherence to bone and the strong interaction with calcium and hydroxyapatite. The chemical nature of R mainly determines the pharmacological properties, such as the mode of action, relative efficacy with respect to degradation and formation of bone, intestinal absorption, biological half life, among others [8, 9, 33].
Ibandronate has been shown to prevent vascular calcification in various animal models, including adenine-induced CRF [13–15, 34]. The prevention of vascular calcification is of special interest in pediatric patients, as these are especially prone to suffer from this complication already at a young age [29, 35]. Moreover, this population only rarely presents with additional "classical" risk factors (e.g. smoking, obesity, and metabolic syndrome), which otherwise are serious confounders. Although the utilization of ibandronate for this purpose is appealing in general, this drug may interfere with endochondral bone formation and longitudinal growth. In fact, growth failure is a serious complication in pediatric CKD patients and may occur even in early stages of CKD [36, 37]. Therefore, we have investigated growth plate morphology, tibial growth rate, and mineral apposition rate in rats with preserved and moderately reduced renal function receiving long-term (10 weeks) ibandronate treatment, with therapy starting at 8 weeks of age. We selected a rather low dose of ibandronate and applied this once weekly. The moderate reduction in kidney function translated into quiet subtle aberrations of growth plate morphology, a (not yet significant) suppression of tibial growth rate, and a discrete reduction of the mineral apposition rate. A clear and significant reduction in each of these parameters was observed in ibandronate-treated controls, whereas in CRF rats an aggravation of the pre-existing pathological conditions was barely detectable. We cannot exclude the possibility that, in rats with more severe renal failure and thus advanced renal osteodystrophy, long-term ibandronate may exert more deleterious effects on bone mineralization. Growth plate morphology was analyzed at the end of a 10-week treatment period, i.e., at an age when growth rates were already declining. Although the duration (10 weeks) of treatment may have masked the early effects of ibandronate on endochondral growth, distinct and significant differences between the ibandronate-treated and non-treated animals were observed. Thus, our findings indicate that ibandronate has the capability to interfere with endochondral ossification and mineralization in the growing skeleton. On the other hand, in this model of mild chronic uremia, the negative effects of ibandronate beyond those induced by the underlying renal failure were marginal. It is rather unlikely that these effects were due to an impaired function of the osteoblasts, since the concentration of ibandronate required to inhibit bone mineralization is several orders of magnitude beyond that required to inhibit bone resorption [9]. Rather, the effects most likely reflect the interference of ibandronate with the growth of hydroxyapatite crystals, i.e. the apposition of new mineral. In fact, the biological action of bisphosphonates is due to both a physicochemical interaction with hydroxyapatite, in which bisphosphonates may act as inhibitors of hydroxyapatite formation, and intracellular biochemical interactions secondary to endocytotic uptake. The latter occur preferentially in osteoclasts, leading unequivocally to apoptosis and thus suppression of bone resorption. However, at the same time, the drug is released, sticking to the skeleton as before and available for another round of uptake and release after the induction of apoptotic cell death. Thus, inhibition of bone resorption is mainly due to the cellular action of bisphosphonates, whereas physicochemical properties are mostly responsible for the interference with bone formation. Several bisphosphonates currently used in the clinical setting have been shown to attenuate the growth of hydroxyapatite crystals in vitro, and there is no reason why this should not happen in vivo [38]. In fact, the significantly reduced mineral apposition rates in ibandronate-treated rats observed in our study support this notion. Etidronate and pamidronate have recently been shown to prevent vascular calcification in uremic rats and, concomitantly, to affect bone formation and mineralization [10, 11]. Although a comparison of these studies, including the present one, is hampered by different experimental designs (e.g. procedures for installation of chronic renal failure, dosage and type of bisphosphonates, and duration of therapy), the results do indicate that the utilization of bisphosphonates in CKD patients interferes with bone mineralization and may substantially inhibit bone turnover. Thus, bisphosphonate treatment is likely to have a bi-phasic effect on vascular calcification: vascular calcification will initially be decelerated or even prevented through a decreased bone resorption, but it will accelerate once adynamic bone has been established and calcium and phosphate are no longer incorporated into the bone, resulting in high circulation levels of these minerals. [12]. Given the long biological half-life, the different relative potencies for inhibition of bone resorption, and the physicochemical properties of bisphosphonates, the duration of treatment and dosage should perhaps be much lower than thus far anticipated.
Finally, clinical data on long-term bisphosphonate usage and bone quality are inconclusive. In patients without CKD, the reduction in bone turnover caused by bisphosphonates contributes to improved mineral apposition and an increase in BMD [33]. However, it remains to be elucidated if long-term bisphosphonate usage results in improved bone quality and resistance to fracture in CKD patients.
In conclusion, ibandronate interferes with tibial growth and bone mineralization even in young rats with normal renal function. Although, bisphosphonates may be a promising therapy for the prevention of ectopic calcifications in CKD, carefully designed pre-clinical studies are required to define the optimal therapeutic window, particularly when these agents are being used in pediatric patients.
References
Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G (2006) Definition, evaluation, and classification of renal osteodystrophy: a position statement from kidney disease: improving global outcomes (KDIGO). Kidney Int 69:1945–1953
Moe SM, Drueke T, Lameire N, Eknoyan G (2007) Chronic kidney disease-mineral-bone disorder: a new paradigm. Adv Chron Kidney Dis 14:3–12
Hruska KA, Saab G, Chaudhary LR, Quinn CO, Lund RJ, Surendran K (2004) Kidney-bone, bone-kidney, and cell-cell communications in renal osteodystrophy. Semin Nephrol 24:25–38
Kiela PR, Ghishan FK (2009) Recent advances in the renal-skeletal-gut axis that controls phosphate homeostasis. Lab Invest 89:7–14
Lund RJ, Davies MR, Brown AJ, Hruska KA (2004) Successful treatment of an adynamic bone disorder with bone morphogenetic protein-7 in a renal ablation model. J Am Soc Nephrol 15:359–369
Hofbauer LC, Brueck CC, Shanahan CM, Schoppet M, Dobnig H (2007) Vascular calcification and osteoporosis–from clinical observation towards molecular understanding. Osteoporos Int 18:251–259
Green JR (2004) Bisphosphonates: preclinical review. Oncologist 9[Suppl 4]:3–13
Drake MT, Clarke BL, Khosla S (2008) Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc 83:1032–1045
Russell RG (2007) Bisphosphonates: mode of action and pharmacology. Pediatrics 119[Suppl 2]:S150–S162
Tamura K, Suzuki Y, Hashiba H, Tamura H, Aizawa S, Kogo H (2005) Effect of etidronate on aortic calcification and bone metabolism in calcitoriol-treated rats with subtotal nephrectomy. J Pharmacol Sci 99:89–94
Lomashvili KA, Monier-Faugere MC, Wang X, Malluche HH, O'Neill WC (2009) Effect of bisphosphonates on vascular calcification and bone metabolism in experimental renal failure. Kidney Int 75:617–625
Neven EG, De Broe ME, D'Haese PC (2009) Prevention of vascular calcification with bisphosphonates without affecting bone mineralization: a new challenge? Kidney Int 75:580–582
Price PA, Faus SA, Williamson MK (2001) Bisphosphonates alendronate and ibandronate inhibit artery calcification at doses comparable to those that inhibit bone resorption. Arterioscler Thromb Vasc Biol 21:817–824
Price PA, Buckley JR, Williamson MK (2001) The amino bisphosphonate ibandronate prevents vitamin D toxicity and inhibits vitamin D-induced calcification of arteries, cartilage, lungs and kidneys in rats. J Nutr 131:2910–2915
Price PA, Roublick AM, Williamson MK (2006) Artery calcification in uremic rats is increased by a low protein diet and prevented by treatment with ibandronate. Kidney Int 70:1577–1583
Persy V, De Broe M, Ketteler M (2006) Bisphosphonates prevent experimental vascular calcification: treat the bone to cure the vessels? Kidney Int 70:1537–1538
Geng Z, Monier-Faugere MC, Bauss F, Malluche HH (2000) Short-term administration of the bisphosphonate ibandronate increases bone volume and prevents hyperparathyroid bone changes in mild experimental renal failure. Clin Nephrol 54:45–53
Tomat A, Gamba CA, Mandalunis P, De Grandi MC, Somoza J, Friedman S, Zeni S (2005) Changes in bone volume and bone resorption by olpadronate treatment in an experimental model of uremic bone disease. J Musculoskelet Neuronal Interact 5:174–181
Haffner D, Hocher B, Müller D, Simon K, Konig K, Richter CM, Eggert B, Schwarz J, Godes M, Nissel R, Querfeld U (2005) Systemic cardiovascular disease in uremic rats induced by 1, 25(OH)2D3. J Hypertens 23:1067–1075
Brkovic D, Seibel M, Juchem R, Linke J, Rohde D, Bauss F (2004) Effect of augmentation cystoplasty on bone metabolism in chronic uremic rats. J Urol 171:921–925
Brkovic D, Linke J, Jakse G, Bauss F (2005) Changes in bone structure after augmentation cystoplasty in chronic uraemic rats. BJU Int 95:1066–1070
Behets GJ, Verberckmoes SC, Oste L, Bervoets AR, Salome M, Cox AG, Denton J, De Broe ME, D'Haese PC (2005) Localization of lanthanum in bone of chronic renal failure rats after oral dosing with lanthanum carbonate. Kidney Int 67:1830–1836
Donath K, Breuner G (1982) A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge-Schliff (sawing and grinding) technique. J Oral Pathol 11:318–326
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR histomorphometry nomenclature committee. J Bone Miner Res 2:595–610
Hunziker EB (1994) Mechanism of longitudinal bone growth and its regulation by growth plate chondrocytes. Microsc Res Tech 28:505–519
Eurell JA, Sterchi DL (1994) Microwaveable toluidine blue stain for surface staining of undecalcified bone sections. J Histotechnol 17:357–359
Cruz-Orive LM, Hunziker EB (1986) Stereology for anisotropic cells: application to growth cartilage. J Microsc 143:47–80
Cobo A, Lopez JM, Carbajo E, Santos F, Alvarez J, Fernandez M, Weruaga A (1999) Growth plate cartilage formation and resorption are differentially depressed in growth retarded uremic rats. J Am Soc Nephrol 10:971–979
Oh J, Wunsch R, Turzer M, Bahner M, Raggi P, Querfeld U, Mehls O, Schaefer F (2002) Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation 106:100–105
Foley RN, Parfrey PS, Sarnak MJ (1998) Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32:S112–S119
Ishitani MB, Milliner DS, Kim DY, Bohorquez HE, Heimbach JK, Sheedy PF 2nd, Morgenstern BZ, Gloor JM, Murphy JG, McBane RD, Bielak LF, Peyser PA, Stegall MD (2005) Early subclinical coronary artery calcification in young adults who were pediatric kidney transplant recipients. Am J Transplant 5:1689–1693
Raggi P, Giachelli C, Bellasi A (2007) Interaction of vascular and bone disease in patients with normal renal function and patients undergoing dialysis. Nat Clin Pract Cardiovasc Med 4:26–33
Toussaint ND, Elder GJ, Kerr PG (2009) Bisphosphonates in chronic kidney disease; balancing potential benefits and adverse effects on bone and soft tissue. Clin J Am Soc Nephrol 4:221–233
Price PA, Omid N, Than TN, Williamson MK (2002) The amino bisphosphonate ibandronate prevents calciphylaxis in the rat at doses that inhibit bone resorption. Calcif Tissue Int 71:356–363
Briese S, Wiesner S, Will JC, Lembcke A, Opgen-Rhein B, Nissel R, Wernecke KD, Andreae J, Haffner D, Querfeld U (2006) Arterial and cardiac disease in young adults with childhood-onset end-stage renal disease–impact of calcium and vitamin D therapy. Nephrol Dial Transplant 21:1906–1914
Haffner D, Schaefer F, Nissel R, Wuhl E, Tonshoff B, Mehls O (2000) Effect of growth hormone treatment on the adult height of children with chronic renal failure. German study group for growth hormone treatment in chronic renal failure. N Engl J Med 343:923–930
Rabkin R, Sun DF, Chen Y, Tan J, Schaefer F (2005) Growth hormone resistance in uremia, a role for impaired JAK/STAT signaling. Pediatr Nephrol 20:313–318
Nancollas GH, Tang R, Phipps RJ, Henneman Z, Gulde S, Wu W, Mangood A, Russell RG, Ebetino FH (2006) Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone 38:617–627
Acknowledgments
The study was funded by a grant (FORUN-program) from the Medical Faculty, University of Rostock. The authors wish to thank Dorothea Gütschow and Katrin Sievert-Kuechenmeister for outstanding technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fischer, DC., Jensen, C., Rahn, A. et al. Ibandronate affects bone growth and mineralization in rats with normal and reduced renal function. Pediatr Nephrol 26, 111–117 (2011). https://doi.org/10.1007/s00467-010-1660-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-010-1660-5