Abstract
Recent years has seen an increasing use of regional citrate anticoagulation in pediatric dialysis. Several approaches have been described for monitoring anticoagulation in the extracorporeal circuit, such as serum citrate levels, post-filter ionized calcium (iCa), and activated coagulation time (ACT). However, no standard recommendations have yet been established for applying any of these parameters, especially for iCa. The objective of this retrospective analysis was to establish adequate coagulation management using post-filter iCa values. Normal values for ACTester-based ACT were established using a group of 64 children who were divided into two subgroups, with one subgroup comprising children without chronic kidney disease or coagulation disorder (age 1.2–17.5 years, median 9.7 years) and one consisting of 32 uremic patients (age 0.6–17.5 years, median 13.7 years). In a second group of 13 patients (aged 7–17 years), all of whom were undergoing high-flux dialysis (HD) with regional citrate anticoagulation (RCA), we assessed 73 post-filter blood samples for ionized calcium and ACT. A receiver operating characteristic graph was used to identify the iCa threshold needed to achieve adequate anticoagulation. Normal values for ACT were 90 s [2 standard deviations (SD) 72–109] in healthy children and 94 s (2 SD 75–113) in the uremic children. There was no statistically significant difference between the groups. In the children undergoing HD with RCA, the post-filter iCa level correlated with ACT (r = −0.94, p < 0.001). A post-filter iCa level of ≤0.30 mmol/l reliably predicted an ACT >120 s. Our citrate protocol [citrate 3% rate (ml/h) ≈ blood flow rate (ml/min) × 2] meets the established criteria with a high sensitivity. Based on these results, we conclude that the post-filter iCa level can be reliably used for the management of extracorporeal anticoagulation with citrate in pediatric HD. We recommend the application of our citrate prescription protocol in the setting of pediatric intermittent hemodialysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
First described by Morita in 1961 [1], regional citrate anticoagulation (RCA) has been used for more than two decades, mainly in continuous renal replacement therapy (CRRT) in patients with a high bleeding risk [2, 3]. Several studies in adults and a number in the pediatric age group have validated the safety and efficacy of this protocol [2–9]. Citrate was used as an anticoagulant in up to 56% of CRRT sessions performed at the North American centers participating in the Prospective Pediatric Continuous Renal Replacement Therapy Registry [10].
Protocols for monitoring an adequate extracorporeal anticoagulation in RCA have been reported, with activated coagulation time (ACT) and post-filter ionized calcium (iCa) level being the most commonly used parameters [2–9]. Standard values for ACT in children are non-existent, and recommendations for ACT in pediatric hemodialysis are mostly empiric and heavily dependent on the method and equipment used [e.g. >120 s for the ACTester (Quest Medical, Allen, TX) and >170 s for the Hemochron (International Technidyne, Edison, NJ)] [11]. If the post-filter iCa is used for coagulation monitoring, the target value varies between ≤0.50 and ≤0.25 mmol/l depending on the particular study [1–9]. Although studies in vitro have demonstrated the association of iCa levels and coagulation time [12, 13], to the best of our knowledge no precise target value based on the anticoagulation measurement of post-filter iCa has been established.
The primary aims of this retrospective analysis were to evaluate the association of post-filter iCa and ACT to provide adequate monitoring of extracorporeal anticoagulation in intermittent high-flux hemodialysis in children and adolescents and also to review our citrate dose protocol. In addition, we wanted to identify a post-filter iCa threshold below which adequate anticoagulation is achieved.
Patients and methods
Two groups of patients were analyzed in this study: (1) 64 patients subdivided into uremic and non-uremic children were evaluated to establish standard values for the ACTester-based ACT; (2) 13 patients who received intermittent hemodialysis (iHD) with RCA were assessed for anticoagulation management by means of a data analysis.
In the non-uremic subgroup, we analyzed the coagulation parameters of 32 patients from our pediatric nephrology out-patient facility as part of the normal screening program, such as patients with hematuria. Thirty-two patients at chronic kidney disease (CKD) stadium V who had coagulation screening as part of the preparation for kidney transplantation were placed into a “uremic” subgroup. The demographic and coagulation data are shown in Table 1.
Intermittent high-flux hemodialysis with RCA was performed in 13 patients. All patients had an increased risk of hemorrhagic complications or overt bleeding and therefore received RCA. Six patients had developed from end-stage renal disease and seven suffered from acute dialysis-dependent kidney injury. Of these 13 patients, six were dialyzed within an intensive care setting. Patient data are given in Table 2.
In the first group of patients we retrospectively compiled demographic data and coagulation parameters, including ACT, by review of the patients’ records. For the ACT measurement, we used the ACTester system with diatomeenpelit (diatomaceous earth, amorphous silcium dioxide) tubes.
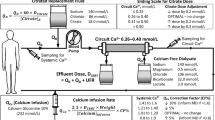
The dialysis modalities in the second group of patients were as follows. The AK 200 (Gambro, Planegg-Martinsried, Germany) pediatric hemodialysis system was used with polysulfone high-flux filters (F×40 or F×60, depending on body-weight; Fresenius, Bad Homburg, Germany). The blood flow rate was 3–5 ml/kg/min but did not exceed 200 ml/min. The ACD-A solution (3% citrate; Baxter Deutschland, Unterschleissheim, Germany) was placed on an external IV-pump and connected to the former ‘heparin line’ of the dialysis circuit. Calcium gluconate 10% (B. Braun AG, Melsungen, Germany) was placed on an IV-pump and connected through a three-way stop cock to the ‘venous’ line of dialysis catheter or shunt needle. A calcium-free concentrate (SK-F 219-0; Fresenius) was used to generate the dialysis fluid. The initial ACD-A infusion rate was set at 3.3% of the blood flow rate, simplified by the equation: citrate 3% rate (ml/h) ≈ blood flow rate (ml/min) × 2.
The calcium solution was started at initiation of RCA at 0.4% of the blood flow rate, simplified by the equation: calcium gluconate 10% rate (ml/h) ≈ blood flow rate (ml/min)/4.
The citrate flow was adjusted to maintain post-filter iCa at ≤0.30 mmol/l. Citrate administration was not adjusted by means of the ACT measured during the treatment. The calcium gluconate substitution was adjusted to maintain the patient’s iCa within the physiological range (1.00–1.30 mmol/l). In addition to monitoring electrolytes and the acid–base balance, we drew 73 post-filter blood samples during the dialysis sessions and analyzed these simultaneously for iCa (ABL blood gas analyzer; Radiometer, Willich, Germany) and ACT (ACTester).
To analyze the data, we retrospectively calculated pre-filter serum citrate levels using the blood flow rate, citrate flow rate, ACD-A citrate concentration (112.9 mmol/l), and the patient’s hematocrit. Statistical analysis was performed using SPSS ver. 16.0 (SPSS, Chicago, IL). Normal distribution was tested using the Kolmogorov–Smirnov test. Normally distributed data are reported as the mean ± standard deviation (SD). To test for significant differences, we used the t test for normal distributed data; in all other cases, the Mann-Whitney–U test was used. For non-linear correlations, we used the Spearman coefficient of correlation; for linear correlations, the Pearson’s coefficient was used. Non-linear regression analysis was used to validate three predictive mathematical models. We used a receiver operating characteristic graph (ROC) to determine a post-filter iCa cut-off level for adequate anticoagulation. A pair of variates was considered positive if the ACT was >120 s. The level of significance was set at p < 0.05.
Results
The mean ACT measured in uremic (healthy) and non-uremic patients was 90 and 94 s, respectively; the two standard deviations of the mean representing normal values were 72–109 and 75–113 s, respectively. The ACTs of the uremic and non-uremic patients were not significantly different. At our center, the empirical ACT range considered to be necessary for hemodialysis had been targeted at 120–180 s. As expected, this ACT target range was found to be significantly beyond the normal range as reported above (p < 0.001).
We found a significant correlation between the post-filter iCa level and the post-filter ACT, with a correlation coefficient of −0.94 (p < 0.001). Three mathematical models were generated with the goal of estimating the ACT from the post-filter iCa level. Non-linear regression analysis showed that the best predictive value was an S-graph function, with an R 2 of 0.89 (p < 0.001). The model’s equation is as follows:
Observed data and the graphs of the three mathematical models are given in Fig. 1.
Relationship of post-filter activated coagulation time (ACT) and ionized calcium levels (iCa). Observed data (open circles) of 13 patients (73 paired measurements) and graphs of three mathematical models established to estimate ACT from the iCA level. Solid line S-graph, dotted line inverse function, broken line exponential function
Based on the correlation of iCa level with ACT, we used a ROC graph (Fig. 2) to calculate the post-filter iCa threshold below which the predicted ACT would reliably be >120 s. A minimal type I error of 0% was found with a post-filter iCa of <0.30 mmol/l; accordingly, a post-filter iCa <0.30 mmol/l was considered to be 100% reliable in predicting an ACT >120 s. With this post-filter iCa level, the type II error was 5.6%. Hence, in about 6% of cases, an ACT >120 s was achieved at iCa levels ≥0.30 mmol/l.
Receiver operator characteristic (ROC) graph denoting an ACT >120 s as the “positive value”; based on the data of Fig. 1
Using our citrate dosing protocol we retrospectively calculated a pre-filter plasma citrate concentration of 4.2–7.2 mmol/l with a median of 5.2 mmol/l. We found that the calculated pre-filter serum citrate levels were correlated with the post-filter iCa level (r = −0.68; p < 0.001).
Using our citrate prescription protocol [citrate 3% rate (ml/h) ≈ blood flow rate (ml/min) × 2], we calculated that 68/73 of the dialysis sessions (93%) started with a post-filter iCa <0.30 mmol/l, thereby meeting the criteria described above. In those patients who started with a post-filter iCa <0.30 mmol/l, the citrate to blood flow ratio did not need to be altered during 57/68 dialysis sessions (78%). Overall, ten of our 13 patients had no alterations of the citrate to blood flow ratio while on dialysis and between dialysis sessions.
In none of the extracorporeal circuits was relevant clot formation found after the dialysis session.
Discussion
Introduced by Hattersley in 1966 [14], the activated coagulation time is an inexpensive, rapid, and easily performable bedside whole blood test for monitoring anticoagulation characterized by repeatable results. The ACT is currently a standard procedure for monitoring anticoagulation in extracorporeal circuits, such as in clinical settings of cardiopulmonary bypass, hemodialysis, and apheresis treatment, and during surgical and interventional procedures requiring anticoagulative treatment, including cardiac and vascular surgery [15–17]. The ACT has also been advocated as a routine preoperative screening test for detecting coagulation disorders [18, 19]. As a whole blood coagulation test, the ACT is affected by different anticoagulants and therefore can be used for monitoring the effects of unfractionated heparin, nafamostat, aprotinin, citrate, and hirudin [20]. It is also affected by certain congenital disorders of coagulation, warfarin, and liver failure [18, 21, 22].
Definitive recommendations for ACTs to achieve adequate anticoagulation are difficult to put into practice due to the manifold techniques (and equipment) used for making the measurement. As such, caution is recommended, and times relative to reference or baseline (pre-dialysis) ACT values are safer to use. In general, times of 1.4- to 2.3-fold the baseline ACT can be considered sufficient for anticoagulation [11, 20]. The lower the blood flow rate, the longer the ACT should be. ACT has been shown to correlate with the duration of dialyzer survival in CRRT [23].
To the best of our knowledge, no reference values for ACTester-based ACT measurements have been described for the pediatric population in the literature. Using our analysis protocol, we determined the normal range of ACT measured by the ACTester in 64 children and found this range to be shorter than normal ranges reported in the literature using other ACT measuring methods ([e.g. 81–133 s [11, 18] or 96–152 s (Hemochron)]. This is easily explained by the different functional principles of ACT measurement and associated varying norm values and, in turn, may also explain the different recommendations for anticoagulation targets found in the literature [11, 20, 24].
Although, the influence of uremia on a patient’s coagulation status has been known about since the 18th century and has been described as bleeding complications in adult chronic hemodialysis patients [25], we found no statistically significant difference between ACT reference values in uremic and non-uremic children and adolescents.
Citrate decreases the free (ionized) serum calcium by forming chelate complexes [1, 8, 9]. Thus, calcium is not available for activation of the tenase complex and the prothrombinase complex of the coagulation cascade [20]. Fibrin generation is thereby inhibited. Citrate also inhibits platelet aggregation. Nuthall et al. found a linear relationship between iCa and serum citrate levels in an animal model of RCA in continuous venovenous hemofiltration [26]. Bakker et al. found a hyperbolic relationship between iCa and measured serum citrate levels in ten adult patients with RCA [27]. They also found that a serum citrate level of 4 mmol/l (3–5 mmol/l) was needed to achieve an iCa <0.40 mmol/l. This result corresponds with our findings. Admittedly, our citrate levels were retrospectively calculated and therefore have to be interpreted with caution.
The work of Ataullakhanov et al. (1994), which is often cited in publications dealing with RCA-anticoagulation, occurred at an iCa <0.5 mmol/l [12]. In our opinion, these findings are out of date. Our own data and the findings of Calatzis et al. show that a much lower iCa is necessary for a prolongation of ACT [13]. Our findings are supported by the work of Opatrný et al. [28]. Their protocol targeted a post-filter iCa at <0.40 mmol/l, but the range actually achieved was 0.35–0.82 mmol/l. At these iCa levels, they found no prolongation of post-filter ACT. The data of Calatzis et al. show an onset of ACT prolongation at iCa levels <0.32 mmol/l [13]. Calcium-dependent anticoagulation seems to work with an "on–off" principle below a certain iCa blood level (Fig. 1). A receiver–operator characteristic graph calculated from our data showed that a post-filter iCa <0.30 mmol/l is needed to reliably achieve a post-filter ACT >120 s. In accordance with the published results of Calatzis et al. [13], we found a hyperbolic relationship between post-filter coagulation time and post-filter iCa, with a high correlation between both parameters. We therefore recommend keeping the post-filter iCa <0.30 mmol/l for adequate anticoagulation in regional citrate anticoagulation, as supported by the recommendations of Ward and Davenport [11, 20]. In our experience the post-filter iCa is a suitable parameter for monitoring anticoagulation of the extracorporeal circuit in RCA in iHD.
Our citrate dosing protocol [citrate flow (ml/h) = blood flow (ml/min) × 2] achieved, at a high percentage, the targets established in this analysis. In the majority of patients and dialysis sessions no adaption of the citrate prescription was necessary during dialysis. Comparable data using the same thresholds are not yet available. We recommend our RCA dosing protocol for pediatric iHD because of its striking simplicity and its reliability in terms of the objectives described above.
Conclusions
Both ACT and post-filter iCA levels can be used for the management of extracorporeal anticoagulation with citrate in high-flux dialysis of children and adolescents. To achieve adequate anticoagulation, we recommend that a post-filter iCa level of <0.30 mmol/l should be targeted as it reliably corresponds to ACT >120 s. Our citrate prescription protocol meets these criteria with high sensitivity and therefore is recommended for pediatric iHD in patients with an increased risk of hemorrhagic complications.
Abbreviations
- ACD:
-
Anticoagulant citrate dextrose
- ACT:
-
Activated coagulation time
- CRRT:
-
Continuous renal replacement therapy
- iCa:
-
Ionized calcium
- iHD:
-
Intermittent hemodialysis
- PTT:
-
Partial thromboplastin time
- RCA:
-
Regional citrate anticoagulation
References
Morita Y, Johnson RW, Dorn RE, Hall DS (1961) Regional anticoagulation during hemodialysis using citrate. Am J Med Sci 242:32–43
Pinnick RV, Wiegmann TB, Diederich DA (1983) Regional citrate anticoagulation for hemodialysis in the patient at high risk for bleeding. N Engl J Med 308:258–261
Mehta R, McDonald B, Aguilar M, Ward DM (1990) Regional citrate anticoagulation for continuous arteriovenous hemodialysis in critically ill patients. Kidney Int 38:976–981
Gubensek J, Buturovic-Ponikvar J, Ponikvar R (2007) Regional citrate anticoagulation for single-needle hemodialysis: a prospective clinical study. Blood Purif 25:454–456
Schneider M, Thomas K, Liefeldt L, Kindgen-Milles D, Peters H, Neumayer HH, Morgera S (2007) Efficacy and safety of intermittent hemodialysis using citrate as anticoagulant: a prospective study. Clin Nephrol 68:302–307
Bunchman TE, Maxvold NJ, Barnett J, Hutchings A, Benfield MR (2002) Pediatric hemofiltration. Normocarb dialysate solution with citrate anticoagulation. Pediatr Nephrol 17:150–154
Bunchman TE, Maxvold NJ, Brophy PD (2003) Pediatric convective hemofiltration: Normocarb replacement fluid and citrate anticoagulation. Am J Kidney Dis 42:1248–1252
Chadha V, Garg U, Warady BA, Alon US (2002) Citrate clearance in children receiving continuous venovenous renal replacement therapy. Pediatr Nephrol 17:819–824
Elhanan N, Skippen P, Nuthall G, Krahn G, Seear M (2004) Citrate anticoagulation in pediatric continuous venovenous hemofiltration. Pediatr Nephrol 19:208–212
Symons JM, Chua AN, Somers MJ, Baum MA, Bunchman TE, Benfield MR, Brophy PD, Blowey D, Fortenberry JD, Chand D, Flores FX, Hackbarth R, Alexander SR, Mahan J, McBryde KD, Goldstein SL (2007) Demographic characteristics of pediatric continuous renal replacement therapy: a report of the prospective pediatric continuous renal replacement therapy registry. Clin J Am Soc Nephrol 2:732–738
Ward DM (1997) The approach to anticoagulation in patients treated with extracorporeal therapy in the intensive care unit. Adv Ren Replace Ther 4:160–173
Ataullakhanov F, Pohilko A, Sinauridze E (1994) Calcium threshold in human plasma clotting kinetics. Thromb Res 75:383–394
Calatzis A, Toepfer M, Schramm W, Spannagl M, Schiffl H (2001) Citrate anticoagulation for extracorporeal circuits: effects on whole blood coagulation activation and clot formation. Nephron 89:233–236
Hattersley PG (1966) Activated coagulation time of whole blood. JAMA 196:436–440
Taneja R, Fernandes P, Marwaha G, Cheng D, Bainbridge D (2008) Perioperative coagulation management and blood conservation in cardiac surgery: a Canadian Survey. J Cardiothorac Vasc Anesth 22:662–669
Slight RD, Buell R, Nzewi OC, McClelland DB, Mankad PS (2008) A comparison of activated coagulation time-based techniques for anticoagulation during cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth 22:47–52
Martindale SJ, Shayevitz JR, D’Errico C (1996) The activated coagulation time: suitability for monitoring heparin effect and neutralization during pediatric cardiac surgery. J Cardiothorac Vasc Anesth 10:458–463
Hattersley PG (1971) The activated coagulation time of whole blood as a routine pre-operative sceening test. Calif Med 114:15–18
Siskin GP, Reiner E, Stainken BF, Dowling K, Dolen EG, Quarfordt S, Albons G (2001) Activated clotting time as a screening test prior to catheter-based cardiovascular procedures. Catheter Cardiovasc Interv 54:191–195
Davenport A (2003) Anticoagulation options for pediatric hemodialysis. Hemodial Int 7:168–176
Rani TR, Gopinath R (2008) An unusual cause of a prolonged activated coagulation time during cardiac surgery: congenital hypofibrinogenemia. J Cardiothorac Vasc Anesth 22:725–726
Chang RJ, Doherty TM, Goldberg SL (1998) How does warfarin affect the activated coagulation time? Am Heart J 136:477–479
Stefanidis I, Hägel J, Maurin N (1995) Influence of coagulation parameters on filter running time during continuous venovenous hemofiltration. Contrib Nephrol 116:145–149
Lord H, Jean N, Dumont M, Kassis J, Leblanc M (2002) Comparison between tinzaparin and standard heparin for chronic hemodialysis in a Canadian center. Am J Nephrol 22:58–66
Hörl WH (2006) Thrombocytopathy and bleeding complications in Uremia. Wien Klin Wochenschr 118:134–150
Nuthall G, Skippen P, Daoust C, Al-Jofan F, Seear M (2002) Citrate anticoagulation in a piglet model of pediatric continuous renal replacement therapy. Crit Care Med 30:900–903
Bakker AJ, Boerma EC, Keidel H, Kingma P, van der Voort PH (2006) Detection of citrate overdose in critically ill patients on citrate-anticoagulated venovenous hemofiltration: use of ionized and total/ionized calcium. Clin Chem Lab Med 44:962–966
Opatrný K, Richtrová P, Polanská K, Wirth J, Sefrna F, Brandl M, Falkenhagen D (2007) Citrate anticoagulation control by ionized calcium levels does not prevent hemostasis and complement activation during hemodialysis. Artif Organs 31:200–207
Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kreuzer, M., Ahlenstiel, T., Kanzelmeyer, N. et al. Management of regional citrate anticoagulation in pediatric high-flux dialysis: activated coagulation time versus post-filter ionized calcium. Pediatr Nephrol 25, 1305–1310 (2010). https://doi.org/10.1007/s00467-010-1483-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-010-1483-4