Abstract
Continuous renal replacement therapy (CRRT) is used to treat critically ill children with acute kidney injury. The effect of CRRT on trace element clearance is poorly characterized. The purpose of this study was to quantify the transmembrane clearance of chromium, copper, manganese, selenium and zinc during continuous venovenous hemodiafiltration (CVVHDF). The transmembrane clearance of trace elements was assessed prospectively in five critically ill children receiving CVVHDF at the pediatric intensive care unit of a tertiary care university hospital. Pre-filter blood and effluent samples were measured for trace element concentrations. Transmembrane clearance of trace elements was calculated, and daily loss of each trace element was determined. Daily trace element loss via CVVHDF was compared with daily standard supplementation of trace elements in pediatric parenteral nutrition. Five patients (age range 23 months to 15 years) with a body weight range of 10.5–53 kg completed the study. The median transmembrane clearance of chromium, copper, manganese, selenium and zinc during CVVHDF was calculated as 0 ml, 0.59 ml, 2.48 ml, 1.22 ml, and 1.90 ml, respectively, per 1.73 m2 body surface area per minute. The calculated CVVHDF losses were substantially smaller than the daily parenteral supplementation for all trace elements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute kidney injury (AKI) is common in hospitalized pediatric patients [1]. Continuous renal replacement therapy (CRRT) is often used to treat critically ill, hemodynamically unstable, pediatric patients with AKI. Compared with intermittent hemodialysis, the continuous nature of CRRT has the advantage of removing fluids and solutes slowly and consistently with less likelihood of causing hemodynamic compromise [2]. CRRT allows the provision of adequate nutrition to meet the requirements of catabolism in pediatric AKI patients because of its expanded capacity to remove fluid and nitrogenous waste products [3]. Although CRRT accommodates the parenteral administration of large volumes of fluid and nutrition, it also removes nutritional solutes and drugs from plasma. Further, the effect of CRRT on trace element clearance is largely unknown and requires further investigation [4–7].
Trace elements are cofactors in enzymatic reactions. The optimal requirements of trace elements in critically ill pediatric patients and those with AKI are unknown. Trace elements, including chromium, copper, manganese, selenium and zinc, are typically added to daily parenteral nutrition in accordance with the daily requirements for intravenously administered trace elements set by the Nutrition Advisory Group of the American Medical Association [8] and the Society of Clinical Nutrition [9]. However, trace element requirements vary with the patient’s nutritional and underlying clinical conditions, where additions or restrictions to the normal trace element intake are required. For instance, zinc and selenium requirements are increased in patients with diarrhea and malabsorption syndromes, due to increased gastrointestinal losses [10]. Copper and manganese restriction is indicated in patients with severe cholestasis, due to their reduced biliary excretion [11].
The clearance of trace elements during CRRT has been investigated in adult AKI patients [5–7, 12]. In published reports, only two used trace element assay technology sensitive enough to detect very low trace element concentrations in ultrafiltrate or dialysate [7, 12]. None of these studies addressed trace element clearance in pediatric patients that were receiving CRRT, and there are no trace element dosing guidelines available for children on CRRT. Factors complicating our understanding of trace element disposition in pediatric CRRT patients are changes in trace element tissue distribution and renal excretion, alteration in plasma protein-binding, changes in oral intake and absorption, dialysate trace element contamination, and loss of trace elements via the hemodiafilter [13–15].
The purpose of this prospective study was to determine the transmembrane clearance of chromium, copper, manganese, selenium and zinc and quantify their daily CRRT loss in relation to amounts received in nutritional replacements in five pediatric patients receiving CRRT.
Materials and methods
Inclusion criteria
Trace element transmembrane clearance was assessed in five critically ill children with AKI treated with continuous venovenous hemodiafiltration (CVVHDF) at C.S. Mott Children’s Hospital, Ann Arbor, MI, USA. AKI in these patients was defined as a rise in serum creatinine (Scr) by at least 25% over baseline, in addition to oliguria or anuria. All five patients were anuric at the time of CRRT initiation. Patients were enrolled from May 2004 until February 2005. Study patients were identified from the pharmacy’s daily list of CRRT patients. The patient database of the University of Michigan Hospitals CareWeb was used for the maintenance of patients’ records. Study investigators obtained informed consent from the patients, patients’ families or legal guardians, and the study was conducted in compliance with the University of Michigan Hospitals’ standards of patient confidentiality according to the Health Information Portability and Accountability Act (HIPAA). The study was approved by the Institutional Review Board of the University of Michigan Medical School (IRBMED). Patients were eligible for the study if they were <18 years of age, weighed > 5 kg dry weight, and were being treated by CVVHDF. Patients were excluded if they required extracorporeal life support or extracorporeal membrane oxygenation (ECMO).
Prescription flow rates, hemofilters, and anticoagulation
A high permeability acrylonitrile (AN-69) hemodiafilter (M60, Gambro, Lakewood, CO, USA) with a surface area of 0.6 m2 and an ultrafiltration coefficient (Kuf) of 16 ml/h per millimeter of mercury for smaller patients with a maximum blood flow rate of 100 ml/min is typically used during CVVHDF treatment of our pediatric patients. A polysulfone hemodiafilter (HF1000, Gambro) with a surface area of 1.1 m2 and a Kuf of 37 ml/h per millimeter of mercury is used for patients who require blood flow rates above 100 ml/min. The Prisma (Gambro) CRRT machine was used for all study patients. Initial effluent (combined ultrafiltrate and dialysate) flow rates were ∼2000 ml/1.73 m2 per hour and were adjusted based on the patient’s volume removal needs. All ultrafiltrate replacement fluids were administered before the filter. The filter choice/set is based upon weight. The AN69 filter set is used for patients <25 kg, and the polysulfone filter set is used for patients weighing 25 kg or more. The priming volume is 100 ml for the AN69 set and 150 ml for the polysulfone set. If the volume of the circuit has been calculated to be less than 10% of the patients blood volume, then the circuit is primed with saline solution. If the circuit is calculated to be 10% or more of the patient’s blood volume then the circuit is primed with blood. This is usually done for any patient of 10 kg or less. For all study patients citrate anticoagulation was used.
Dosing of trace mineral supplementation
Patients weighing < 30 kg receive our standard trace element preparation, dosed intravenously at 0.2 ml/kg per day. This formulation contains the following ingredients (per 0.2 ml): zinc 0.2 mg, copper 0.02 mg, manganese 5 μg, chromium 0.2 μg, and selenium 3 μg. Patients weighing 30 kg or more receive 1 ml of MTE-5 for adults. This formulation contains (per 1 ml): zinc 5 mg, copper 1 mg, manganese 0.5 mg, chromium 0.01 mg, and selenium 0.06 mg.
Sample collection
All samples were obtained through trace metal-free stainless steel needles (Becton Dickinson Laboratories, Franklin Lakes, NJ, USA). Samples were obtained from the pre-filter port (A) and the effluent (EF) from the effluent sampling ports of the CRRT circuits. Samples were placed on ice immediately and transferred to the research laboratory at the University of Michigan College of Pharmacy. Each EF sample was placed into trace metal-free Falcon® tubes (Becton Dickinson Labware) and stored at −80°C until required for assay. Blood samples were transferred to Falcon® tubes and centrifuged at 3,000 r.p.m.. for 30 min. The plasma was transferred by metal-free pipette into Falcon® tubes and stored at −80°C until required for assay.
Assay
Plasma samples were analyzed for total content of chromium, copper, manganese, selenium and zinc at the University of Notre Dame, South Bend, IN, USA, using inductively coupled plasma–mass spectrometry (ICP-MS). The samples were analyzed according to previously described procedures [7, 16–19], using a Vacuum Generators Elemental PlasmaQuad model PQ2.
Single-element solutions (Inorganic Ventures, Lakewood, NJ, USA) were used to prepare calibration and internal standard solutions. Analyses were performed using an external calibration procedure. Scandium (Sc), cobalt (Co), gallium (Ga), and yttrium (Y) were used as internal standards for matrix and instrument drift corrections. A procedural blank was analyzed to check for any contribution from the reagents and laboratory environment. The reference materials SRM-8414 (bovine muscle) and SRM-1577b (bovine liver) were digested and analyzed so that they were identical to the unknown for quality control purposes. The assay detects trace elements at the sub-nanogram per gram level.
Calculations
Trace element loss was determined by the calculation of an extraction coefficient (EC) and transmembrane clearance of each element. Trace elements and urea EC and transmembrane clearance equations for CVVHDF were:
Extraction coefficient (EC) = EF/A
CVVHDF transmembrane clearance = EC x (Qd + Quf) where A is the solute concentration in the plasma obtained from the pre-filter port, EF is the solute concentration in the effluent/spent dialysate obtained from the effluent/spent dialysate port, Quf is the ultrafiltrate production rate and Qd is the dialysate flow rate.
Once transmembrane clearance had been assessed, the total daily loss of each trace element during CVVHDF was calculated from this equation:
We compared the calculated daily loss of trace elements with the amount of a trace element supplement prescribed parenterally for each patient to determine whether supplementation rates exceeded the transmembrane trace element losses. The results are presented as median values because four trace element concentrations in the effluent were below the detection limit of the assay.
Results
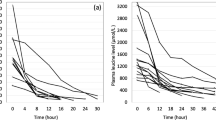
Samples were obtained from five study patients from the pediatric intensive care unit (PICU). Table 1 lists the demographic profiles of the study patients. As is the case in most PICUs, there was a wide range in patient weight and size and, consequently, CVVHDF orders. All patients were receiving parenteral nutrition containing trace element supplementation. The doses of such supplementations were prescribed by the primary medical team, according to pharmacy protocols for parenteral nutrition, and were based on patient weight.
Table 2 depicts the plasma concentrations of the five trace elements studied. As has been observed in critically ill adult CRRT patients [7], considerable variation was seen in these values. Trace element EF concentrations were also assessed. However, chromium EF concentrations were below the lower detection level of the assay in three of the five patients, and, in one subject, the plasma manganese concentration was below the lower detection limit of the assay (less than 1 ng/g). Consequently, the calculated EC in these instances was zero. Because the calculated EC was zero in these instances, the calculated transmembrane clearance was also zero. While it is likely that some chromium and manganese did cross the hemodiafilter membrane, the amount cleared was insignificant, given the exquisite sensitivity of the assay.
Calculated trace element EC and subsequent transmembrane clearances appear in Tables 3 and 4. From these transmembrane clearances, a daily loss of trace elements during CVVHDF was calculated and compared with the parenteral trace element supplementation prescribed daily for each subject (Table 5).
For all five patients and for all the trace elements studied, the amount of trace element supplemented greatly exceeded that removed by CVVHDF. The daily supplemented trace elements that were removed by CVVHDF never exceeded 20%, and, in most cases, they were less than 5% of the corresponding parenteral trace element supplement.
Discussion
To our knowledge, this is the first time that the plasma concentrations of trace elements in critically ill AKI pediatric patients have been reported. This report is a case series of five patients that attempts to quantify the removal of trace elements by CRRT in children and compare these values with typical daily doses of trace elements in parenteral nutrition. The use of various forms of CRRT in children is increasing [20], but studies of CRRT clearance of solutes such as nutritional factors and drugs are few in this patient population.
Previous studies of CRRT nutrition in children have examined amino acid loss [21] but not that of trace elements. Many of the studies of trace element clearance during CRRT that have been conducted in adults [5, 6] have found little, if any, trace element loss by CRRT, but this might have been due to the relatively insensitive assay techniques used. In adults, Berger et al. detected copper, selenium and zinc in CVVHDF effluent, using ICP-MS, but they did not report manganese or chromium values [12]. Klein and colleagues more recently reported finding boron, manganese, nickel, selenium and silicon in effluent from adults receiving either continuous venovenous hemofiltration or continuous hemodialysis, but they did not measure other trace elements [22].
We conducted this prospective series using the more sensitive ICP-MS assay to determine whether the reported low clearance rate of trace elements during CRRT was due to assay limitations of the previous studies or actual low transmembrane clearance of trace element. Our results suggest that the latter explanation is more likely. ICP-MS such as that used in our study can detect trace elements at less than 1 ng/g concentrations, and even at this level of sensitivity, chromium could not be detected in the effluent of three patients and manganese could not be detected in the EF of another.
We recently published a similar study conducted on ten critically ill adults receiving CVVHDF [7]. The results of that trial show some remarkable similarities to the results of this one. For example, trace element concentrations in the plasma of critically ill adults were similar to those seen in this study of children, with the possible exception of zinc. Zinc concentrations in the ten adult patients [mean ± standard deviation (SD) 950 ± 220 ng/g] were 40% higher than those observed in this study. The trace element ECs in our previous study of adults and in this study of children also were very consistent.
In this study, all calculated trace element ECs were ≤ 0.1. This indicates that little of the trace elements had crossed the hemodiafilter membrane. These values are consistent with those reported in critically ill adults treated with CRRT [5–7]. Despite the fairly high effluent rates used in our study (mean ± SD 52.0 ± 5.7 ml/min per 1.73 m2 body surface area), the median transmembrane clearance was relatively low (<3 ml/min) for all trace elements studied.
For low molecular weight solutes such as trace elements, EC and, subsequently, transmembrane clearance for any solute are primarily functions of plasma protein binding. Trace elements are highly protein-bound in healthy individuals. In patients with renal failure some trace elements have altered plasma protein binding [23]. The fraction of the trace element that is not protein-bound can be inferred from the measured EC [24]. In our study, all ECs ranged from 0–0.1, suggesting that the trace element fraction that was not protein-bound ranged from 0–10%. Many factors may affect trace element plasma protein binding in critically ill patients with AKI. For example, acute inflammation alters alpha-2 globulin expression and other trace element binding proteins [23]. Certain drugs also exhibit lower plasma protein binding rates in patients with critical illness and renal failure than in healthy individuals [25, 26].
The dose of trace element supplementation used in our PICU is dependent on the patient’s weight. Because of this dosing scheme, patients 2, 3 and 4 received individualized doses of trace elements, while patients 1 and 5 received the same standard dose. Regardless of this dosing regimen, the results were the same. In all cases, the daily parenteral amounts of trace element supplements greatly exceeded the amounts removed by CVVHDF. These results mirror our findings in adult CRRT patients [7].
The possibility of manganese accumulation is concerning. Magnetic resonance imaging of patients receiving long-term parenteral nutrition (PN) has shown manganese to accumulate in patients with cholestasis. Manganese toxicity is manifested by neurological toxicity, including tremor, gait, headache, confusion, somnolence, weakness, muscle rigidity [27]. Because manganese is primarily eliminated via the bile, further manganese accumulation may occur in patients with cholestasis, thereby increasing the risk for manganese toxicity in these patients [28].
Although copper toxicity has not been reported in patients receiving PN, higher accumulations of copper in the liver have been found in cholestatic patients receiving long-term PN [29]. Copper may AKI in the liver and cause subclinical toxicities, even without a significant increase in serum copper concentrations.
Chromium is excreted primarily by the kidneys. Although chromium toxicity has not been reported in patients receiving PN or those with AKI, chromium is a contaminant of PN ingredients (e.g. amino acids, dextrose, electrolytes, intravenous lipid emulsions), and its supplementation in PN may not be necessary to maintain normal serum chromium concentrations [30, 31].
Serum zinc concentrations may vary between patients, depending on intake, losses, and tissue distribution. Physiological stress (e.g. critical illness) increases the renal filtration and tissue redistribution of zinc. Intestinal zinc losses are increased in patients with diarrhea, short bowel syndrome, and high output fistulas, and patients with severe burns may also have increased losses of zinc through the wounds [29].
The primary limitation of this report is the relatively small number of enrolled patients. Although the ages of these patients ranged from 2–15 years, it may be difficult to generalize the data from only five study patients. Owing to this age range and our institution’s CRRT and nutritional protocols, this led to our using different hemodiafilters, flow rates (although they were normalized to body surface area), and trace element doses. Patient 3 weighed 10.5 kg at the time of the study and consequently received a blood prime when CVVHDF was instituted. The blood and effluent samples were obtained 23 h after the CVVHDF circuit had been started. It is possible that the concentrations of serum trace elements were influenced by the primed blood. However, the EC that was calculated should not have been affected by the concentrations of the serum trace elements or by the source of the blood measured.
Conclusion
Critically ill pediatric patients treated with CVVHDF experience minimal losses of chromium copper, manganese, selenium and zinc in the effluent, similar to our findings in adult CRRT patients [7]. Daily standard supplementation of trace elements in parenteral nutrition exceeds the amounts of trace elements cleared by CVVHDF, even at effluent rates as high as 60 ml/min per 1.73 m2 body surface area. Although abnormal intake or losses of trace elements in specific disease states are well documented, there are no specific guidelines to adjust trace element supplementation to account for the increased or decreased serum trace element concentrations. Given that typical trace element supplementation rates greatly exceed the CRRT elimination rates, it may be prudent to monitor trace element serum concentrations periodically to evaluate the possibility of trace element accumulation, particularly for patients on long-term CRRT.
References
Hui-Stickle S, Brewer ED, Goldstein SL (2005) Pediatric ARF epidemiology at a tertiary care center from 1999–2001. Am J Kidney Dis 45:96–101
Joy MS, Matzke GR, Armstrong DK, Marx MA, Zarowitz BJ (1998) A primer on continuous renal replacement therapy for critically ill patients. Ann Pharmacother 32:362–375
Wooley JA, Btaiche IF, Good KL (2005) Metabolic and nutritional aspects of acute renal failure in critically ill patients requiring continuous renal replacement therapy. Nutr Clin Pract 20:176–191
Nakamura AT, Btaiche IF, Pasko DA, Jain JC, Mueller BA (2004) In-vitro clearance of trace elements via continuous venovenous hemofiltration. J Ren Nutr 14:214–219
Story DA, Ronco C, Bellomo R (1999) Trace element and vitamin concentrations and losses in critically ill patients treated with continuous venovenous hemofiltration. Crit Care Med 27:220–223
Klein CJ, Moser-Veillon PB, Schweitzer A, Douglass LW, Reynolds HN, Patterson KY, Veillon C (2002) Magnesium, calcium, zinc, and nitrogen loss in trauma patients during continuous renal replacement therapy. JPEN J Parenter Enteral Nutr 26:77–93
Churchwell MD, Pasko DA, Btaiche IF, Jain J, Mueller BA (2007) Trace element removal during in vitro and in vivo continuous hemodialysis. Nephrol Dial Transplant 22:2970–2977
American Medical Association, Nutrition Advisory Group (1979) Guidelines for essential trace element preparations for parenteral use. JAMA 241:2051–2054
Greene HL, Hambidge KM, Schanler R, Tsang RC (1988) Guidelines for the use of vitamins, trace elements, calcium, magnesium, and phosphorus in infants and children receiving total parenteral nutrition: report of the subcommittee on pediatric parenteral nutrient requirements from the committee on clinical practice issues of the American Society for Clinical Nutrition. Am J Clin Nutr 48:1324–1342
McLain CJ (1981) Trace metal abnormalities in adults during hyperalimentation. JPEN J Parenter Enteral Nutr 5:424–429
McLain CJ (1985) Zinc metabolism in malabsorption syndromes. J Am Coll Nutr 4:49–64
Berger MM, Shenkin A, Revelly JP, Roberts E, Cayeux MC (2004) Copper, selenium, zinc, and thiamine balances during continuous venovenous hemodiafiltration in critically ill patients. Am J Clin Nutr 80:410–416
Zima T, Mestek O, Nemecek K, Bartova V, Fialova J, Tesar V, Suchanek M (1998) Trace elements in hemodialysis and continuous ambulatory peritoneal dialysis patients. Blood Purif 16:253–260
Milly K, Wit L, Diskin C, Tulley R (1992) Selenium in renal failure patients. Nephron 61:139–144
Churchwell MD, Btaiche IF, Pasko DA, Mueller BA (2008) An understanding of trace metal physiology as an aid to interpretation of clearance studies by artificial organs. Nephrol Dial Transplant 23:1462–1463
Jain JC, Neal CR, Seidler JA (2000) Assessing the accuracy of analysis for low level elemental composition of blood by ICP-MS. Proceedings of the 46th International Conference on Analytical Sciences and Spectroscopy, p 26 (abstract)
Jain JC, Neal CR, Seidler JA (2000) Trace elements analysis of human blood samples by ICP-MS. Proceedings of Pittsburgh Conference, 188ZP (abstract)
Mishra S, Sabbah HN, Jain JC (2003) Reduced Ca2+-calmodulin-dependent protein kinase activity and expression in IV myocardium of dogs with heart failure. Am J Physiol Heart Circ Physiol 248:876–883
University of Notre Dame ICP-MS Analytical Research Facility Dept. Civil Eng. & Geological Sciences, University of Notre Dame, Notre Dame, IN 46556-0767. Available at https://doi.org/www.nd.edu-icpmslab
Symons JM, Chua AN, Somers MJ, Baum MA, Bunchman TE, Benfield MR, Brophy PD, Blowey D, Fortenberry JD, Chand D, Flores FX, Hackbarth R, Alexander SR, Mahan J, McBryde KD, Goldstein SL (2007) Demographic characteristics of pediatric continuous renal replacement therapy: a report of the prospective pediatric continuous renal replacement therapy registry. Clin J Am Soc Nephrol 2:732–738
Maxvold NJ, Smoyer WE, Custer JR, Bunchman TE (2000) Amino acid loss and nitrogen balance in critically ill children with acute renal failure: a prospective comparison between classic hemofiltration and hemofiltration with dialysis. Crit Care Med 28:1161–1165
Klein CJ, Nielsen FH, Moser-Veillon PB (2008) Trace element loss in urine and effluent following traumatic injury. JPEN J Parenter Enteral Nutr 32:129–139
Diskin CJ (1986) Plasma selenium levels in renal failure. Nephron 44:155–156
Rumpf KW, Rieger J, Doht B, Ansorg R, Scheler F (1977) Drug elimination by hemofiltration. J Dial 1:67–68
Boucher BA, Rodman JH, Jaresko GS, Rasmussen SN, Watridge CB, Fabian TC (1988) Phenytoin pharmacokinetics in critically ill trauma patients. Clin Pharmacol Ther 44:675–683
Edwards DJ, Lalka D, Cerra F, Slaughter RL (1982) Alpha 1-acid glycoprotein concentration and protein binding in trauma. Clin Pharmacol Ther 31:62–67
Fell JME, Reynolds AP, Meadows N, Khan K, Long SG, Quaghebeur G, Taylor WJ, Milla PJ (1996) Manganese toxicity in children receiving long-term parenteral nutrition. Lancet 347:1218–1221
Fitzgerald K, Mikalunas V, Rubin H, McCarthey R, Vanaqunas A, Craig RM (1999) Hypermanganesemia in patients receiving total parenteral nutrition. JPEN J Parenter Enteral Nutr 23:333–336
Btaiche IF (2005) Chronic complications associated with parenteral nutrition. In: Schumock G, Brundage D, Chessman K, Dunsworth T, Fagan S, Kelly W, Rethburn R, Richie D, Semla T Vasquez E, Zarowitz B (eds) Pharmacotherapy self-assessment program (PSAP-V), 5th edn. The American College of Clinical Pharmacy, Kansas City, MO, pp 163–180
Pluhator-Murton MM, Fedorak RN, Audette RJ, Marriage BJ, Yatscoff RW, Gramlich LM (1999) Trace element contamination of total parenteral nutrition. JPEN J Parenter Enteral Nutr 23:222–227
Moukarzel AA, Song MK, Buchman AL, Vargas J, Guss W, McDiarmid S, Reyen L, Ament ME (1992) Excessive chromium intake in children receiving total parenteral nutrition. Lancet 339:385–388
Jacob RA, Milne DB (1993) Biochemical assessment of vitamins and trace metals. Clin Lab Med 13:371–385
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
This study was funded in part by a grant from the University of Michigan College of Pharmacy Clinical Research Resources Fund.
Rights and permissions
About this article
Cite this article
Pasko, D.A., Churchwell, M.D., Btaiche, I.F. et al. Continuous venovenous hemodiafiltration trace element clearance in pediatric patients: a case series. Pediatr Nephrol 24, 807–813 (2009). https://doi.org/10.1007/s00467-008-1083-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-008-1083-8