Abstract
Our aim was to report the effect of two treatment regimens in 43 cases of severe Henoch–Schönlein nephritis (HSN) and immunoglobulin A nephritis (IgAN) (24 HSN, 19 IgAN). Group A, 11 HSN and 7 IgAN, 88% with an International Study of Kidney Disease in Children (ISKDC) biopsy grade ≥ III and severe clinical features, were treated with corticosteroids, cyclophosphamide (CYC-P) and angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (ACEi/ARB). Group B, 12 HSN and 13 IgAN, 72% with biopsy findings as above and 52% with severe clinical features, were treated with ACEi/ARB ± corticosteroids. The outcome classification was: (a) healthy; (b) mild proteinuria, normal glomerular filtration rate (GFR); (c) active renal disease; (d) chronic renal failure. Twenty-six patients had a good outcome (a + b). The 17 children with poor outcome (c + d) had lower GFR at onset and at follow-up, higher albumin excretion at follow-up, and higher percentage of segmental glomerulosclerosis in the renal biopsy, than those with good outcome. Treatment with corticosteroids, CYC-P and ACEi/ARB was effective in increasing GFR, reducing proteinuria and decreasing the disease activity index. The proteinuria had decreased at follow-up in both groups. In group A, GFR increased and histopathological activity index declined after treatment. The outcome did not differ between groups A and B. The effects of treatment did not differ between HSN and IgAN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The management of severe Henoch–Schönlein nephritis (HSN) and immunoglobulin A nephritis (IgAN) is still controversial. Severe cases are rare, but the morbidity rate among these patients is high. Severe disease is defined according to the clinical features and duration of proteinuria [1] and renal biopsy findings showing a high proportion of glomeruli with crescents or sclerosis as well as interstitial inflammation or fibrosis [2–5].
Various regimens of treatment in severe HSN and IgAN have been described, such as intravenous administration of methylprednisolone (MP) followed by oral treatment with corticosteroids, or corticosteroids in combination with azathioprine (AZA) or cyclophosphamide (CYC-P), with or without anticoagulants, with or without plasmapheresis, cyclosporine A (CyA) or mycophenolate mofetil [6–21]. Treatment with angiotensin-converting enzyme inhibitors (ACEis) [22] with or without the combination of angiotensin II receptor blockers (ARBs) [23] in both normotensive and hypertensive patients has been shown to be effective in preserving glomerular filtration rate (GFR) and reducing proteinuria in IgAN patients.
The aim of this study was to describe the effects of treatment of two groups of patients. Group A had severe disease and were treated with corticosteroids in combination with CYC-P and ACEi /ARB, whereas group B consisted of patients both with milder and severe disease. All group B patients were treated with ACEi/ARB with or without corticosteroids.
Materials and methods
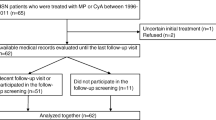
The study was longitudinal and retrospective. We examined 43 children (25 boys), of whom 24 had HSN (14 boys) and 19 IgAN (11 boys), admitted to the department of Pediatric Nephrology at the Karolinska University Hospital, Hudddinge, between May 1990 and December 2005. Our clinic is the larger of two tertiary centers for children with kidney diseases in Sweden. Thus, the patients were mainly referral patients with a severe clinical presentation at onset. Their median age at onset was 12.0 (4.1–17.7) years, and they were followed for a median of 3.1 (0.25–10.5) years. The age at onset did not differ significantly between the groups.
In Group A there were 18 patients (11 HSN, 7 IgAN), of whom 17 had renal biopsy findings according to the International Study of Kidney Disease in Children (ISKDC) of ≥ grade III, in combination with nephrotic proteinuria at onset, and all except one had severe clinical features. They were initially treated intravenously with MP 30 mg/kg for three alternate days. Twelve patients were also treated orally with prednisolone at a starting dose of 1 mg/kg daily, which, 1 month later, was changed to alternate-day dosing and slowly tapered thereafter. The duration of the oral treatment with corticosteroids was a median 0.6 (0.4–3.1) years. After MP, the regimen also included intravenous treatment with CYC-P 500–750 mg/m2 body surface area, 1–3 pulses (14 patients), or six pulses monthly (2 patients), or oral administration of CYC-P 2 mg/kg daily for 8 weeks (2 patients). All patients received ACEis/ARBs.
Group B consisted of 25 patients (13 HSN, 12 IgAN) of whom12 had severe clinical features. Eighteen patients had undergone a biopsy showing ISKDC grade ≥ III, but only five of these 18 had nephrotic proteinuria. Five patients had biopsy findings showing ISKDC grade II, and two patients had ISKDC grade I. All 25 patients were treated with ACEis/ARBs with (15 patients) or without (10 patients) corticosteroids. The duration of the latter medication was a median 0.3 (0.02–0.7) years.
The recommendations of treatment have changed over time. In group A only 3/18 patients had been treated before 1998, compared with 6/25 in group B. At the time, aggressive treatment with immunosuppression not commonly used.
The patients were classified into five categories according to their renal manifestations at onset and at biopsy [24]: (1) micro- or macroscopic hematuria; (2) persistent mild proteinuria (<1 g/l or urine albumin/creatinine ratio (Ua/c) < 200 mg/mmol) ± hematuria; (3) nephritic syndrome (moderate proteinuria (Ua/c≥ 200–400 mg/mmol), decreased GFR, hematuria and/or hypertension [25]); (4) nephrotic syndrome (urinary albumin excretion >40 mg/h per square meter body surface area or Ua/c ≥ 400 mg/mmol, serum albumin <25 g/l); (5) mixed nephritic/nephrotic syndrome at onset. Classes 1 and 2 were considered as mild clinical features, and classes 3–5 as severe. Severe features at onset were present in 66% of group A patients and 48% of group B patients, while, at the time of the first renal biopsy, the corresponding values were 94% and 48%, respectively.
On admission, all patients had undergone a renal function test and a renal biopsy at a median 0.2 years after onset. Nine patients (eight from group A, one from group B; 7/9 HSN, 2/9 IgAN) underwent a second renal biopsy at a median 1.5 years from onset, so that the effect of treatment could be assessed or because of progression of the disease. The GFR and effective renal plasma flow (ERPF) were determined by urinary clearances of inulin and para-amino-hippurate (PAH) during water diuresis [26]. Fifty children with no renal disease served as controls (Table 1).
The outcomes were categorized as: (a) normal and minor urinary abnormalities; this category included both healthy patients without urinary abnormalities and those with microalbuminuria (Ua/c 2.5–25 mg/mmol) with or without hematuria (b) Persistent mild proteinuria and GFR ≥ 94 ml/min per 1.73 m2 (two standard deviations below the mean value of the controls); (c) active renal disease (i.e. hypertension with mean arterial pressure (MAP) > 95th percentile and/or Ua/c ≥ 200 mg/mmol and/or GFR 40–94 ml/min per 1.73 m2; (d) chronic renal failure (CRF) (GFR ≤ 40 ml/min per 1.73 m2) or end-stage renal disease (ESRD, requiring dialysis and/or renal transplantation). Categories a and b were considered as good outcomes and categories c and d as poor outcomes.
All renal biopsies were examined by the same pathologist (M.P.S.) and classified in accordance with the recommendations of the ISKDC [27]. The specimens were also scored for signs of activity and chronicity [6, 28]. Our modified scoring system was based on mesangial matrix expansion/mesangial proliferation (0–3) and percentage of glomeruli with crescent formation (0–3) as indicators of activity, and on percentage of global glomerular sclerosis (GGS) and segmental glomerulosclerosis (SGS) (0–2) and extent of interstitial fibrosis (0–3) as indicators of chronicity. The scoring of interstitial fibrosis was doubled, due to its impact on progressive histological damage [4, 29, 30].
Statistical analysis
Statistica 7.0 (Statsoft Inc., Trulsa USA) was used for all calculations. All data were expressed as mean (standard deviation) or median (minimum–maximum) if not normally distributed. Comparisons between groups were made with Student’s t-test or Mann–Whitney U test, and repeated measurements with Wilcoxon’s matched pairs test. A chi-square test was used for comparisons of proportions. P < 0.05 was considered statistically significant.
Results
There were no differences in any variable [GFR, ERPF, serum albumin, MAP, Ua/c, urine immunoglobulin G/creatinine ratio (UIgG/c)] at any time point between IgAN and HSN patients. Figure 1 illustrates the Ua/c during follow-up. Thus, we have pooled the results from both disease populations.
Group A (n=18) treatment with MP and CYC-P in combination with ACEi/ARB
After three pulses of MP intravenously, the patients’ Ua/c ratios decreased from a median of 739 (149–3335) mg/mmol to 293 (77–1647) mg/mmol, P = 0.0012 (Table 1). After the patients had received additional CYC-P, their Ua/c ratios decreased further to 99 (2–1094) mg/mmol, P = 0.00002 compared with the first visit. This effect was maintained at 1 year after start of treatment and at the last visit. Figure 1a shows that the effects of treatment were similar in IgAN and HSN patients. UIgG/c decreased from a median of 29 (0.1–69) mg/mmol to 7 (1–48) mg/mmol, P = 0.022, after treatment with MP and CYC-P, and this response was maintained at 1 year and at the last visit. Serum albumin increased significantly through the follow-up (Table 1). GFR increased from a median 77 (4–120) ml/min per 1.73 m2 to 105 (8–133) ml/min per 1.73 m2 after treatment, P = 0.02, and remained normal during follow-up (Table 1). ERPF (data not shown) was normal at onset and did not change significantly during follow-up. MAP was normal at onset and did not change during the follow-up.
Group B (n=25) treatment with ACEi/ARB with or without corticosteroids
Ua/c decreased and serum albumin increased significantly during follow-up (Table 1). Figure 1b shows that the effects of treatment on Ua/c were similar in HSN and IgAN patients. However, patients treated with ACEi/ARB and corticosteroids had a higher Ua/c at first visit, a median 391 (44–1,263) mg/mmol, than patients treated with ACEi/ARB alone, a median 44 (4–778) mg/mmol, P = 0.012. The ACEi/ARB- and corticosteroid-treated group showed a greater fall in Ua/c (a median decrease of 286 mg/mmol, 73% of the first value) than the group not treated with corticosteroid (a median decrease in Ua/c of 27 mg/mmol, 61% of the first value). GFR was unchanged during follow-up.
Renal biopsy
ISKDC ≥ grade III was found in 94% of patients in group A and in 72% in group B. Group A patients had significantly more crescents, a median 22 (0–67)%, than group B, a median 0 (0–65)%, P = 0.0007, and the mean activity index was 2.72 ± 0.75 in group A, significantly higher than the 1.64 ± 1.29 in group B, P = 0.0027.
In the nine patients that had undergone repeat biopsy, comparisons between the first and second renal biopsy showed that the activity index had declined from 3.11 ± 0.8 to 1.55 ± 1.5, P = 0.0008. On the other hand, the chronicity index had increased from 2.11 ± 1.8 to 4.33 ± 2.2, P = 0.008, with the SGS having increased from a median of 11% to 31%, P = 0.049, and the GGS from a median 0% to 20%, P = 0.036.
Patients who developed end stage renal disease
The rate of development of ESRD was 14% (four IgAN and two HSN). Table 2 shows the clinical parameters in the six patients (four boys) who developed ESRD in comparison with the 37 who did not. In summary, the ESRD patients had lower GFR and higher Ua/c and U IgG/c at the first visit and at the follow-up visits than did the group that did not have ESRD. All ESRD patients had been over 9 years old at onset, and all except one patient had severe features at onset. The renal biopsy results showed ISKDC grade III in two patients, ISKDC IV in three patients and ISKDC V in one patient. The six patients who had progressed to ESRD had higher a percentage of SGS (median 33) in the first biopsy than did the patients without ESRD (median 8), P = 0.014.
Outcome
Of the 43 patients, 26 (60%) had a good outcome (9/18 in group A, 17/25 in group B) and 17 (40%) had a poor outcome (9/18 in group A, 8/25 in group B). A total of 18 patients (42%) had a complete recovery (7/18 in group A, 11/25 in group B) and were healthy at the last visit. Age at onset did not predict outcome.
The clinical picture at onset progressed in 14 patients to the time of the first renal biopsy. All five patients with a primary presentation of hematuria had developed proteinuria at the time of renal biopsy, and seven patients with mild proteinuria at onset had progressed to nephritic, nephrotic or nephritic–nephrotic syndrome at renal biopsy, and an additional two patients from nephritic or nephrotic syndrome to a mixed form. In view of this progressive worsening of clinical features between onset and the time of renal biopsy, we have chosen to present the outcome in relation to clinical features at the time of renal biopsy (Table 3). Sixteen of 29 patients (55%) with severe clinical features had a good outcome compared with 10/14 (71%) of the patients with mild clinical features [P not significant (n.s.)].
Table 3 also gives the outcome in relation to biopsy findings. The duration of the disease before the biopsy did not differ between the group with good (1.4 ± 2.4 years) and with poor (0.4 ± 0.75 years, P = 0.058) outcome. Of all the renal biopsies, 81% showed advanced findings (ISKDC ≥ III). Nineteen of 35 (54%) of these had a good outcome in comparison with 7/8 (87%) with milder renal biopsy findings (ISKDC < III), P = n.s.. Thus, neither clinical features at renal biopsy nor renal biopsy grade significantly predicted outcome. However, patients with poor outcome had significantly lower GFR at the first visit and lower GFR, higher MAP and Ua/c, and lower serum albumin (S-albumin) 1 year after treatment than did those with a good outcome. Comparing renal biopsy findings between the groups we found a higher percentage of SGS and a higher amount of interstitial fibrosis and interstitial inflammation in the group of patients with poor outcome (Table 4).
There was no significant difference in outcome between the HSN group and the IgAN group. Fifteen of 24 (63%) patients in the HSN group and 11/19 (58%) in the IgAN group had a good outcome. Neither the clinical features nor the ISKDC grading could predict outcome in these two groups.
Discussion
Severe HSN and IgAN in childhood are not common, and there is no consensus on treatment strategies. The aim of this study was to describe our experience at a single center of the effects of treatment in 43 cases of severe HSN and IgAN in a 10-year follow-up study. The study was retrospective and, therefore, has its limitations. The patients’ data were retrospectively analyzed and grouped according to the treatment given. The study groups both differed and overlapped in the severity of clinical features and renal biopsy morphology, and therefore no comparisons to prove efficacy of the different treatments can be made. The treatment tradition has also been changed over time. However, some important observations can be made. Intravenous treatment with MP was associated with a decline in Ua/c, and, after additional oral treatment with corticosteroids and CYC-P in combination with ACEis and ARBs, GFR increased and both Ua/c and UIgG/c decreased further. In the group not treated with CYC-P, the Ua/c was also reduced, while the GFR remained unchanged through the follow-up.
Although several questions are left unsolved regarding the indication and timing of the treatment, many investigators emphasize the importance of early treatment in severe IgAN and HSN, as the morbidity rate is high among patients with ISKDC grades ≥ III and nephrotic range proteinuria [11, 17, 28, 31]. We found a decrease in proteinuria and an increase in GFR following the corticosteroid treatment, and, in group B, 68% of the patients had a good outcome at the last visit. The decline in proteinuria and preservation of GFR after treatment with corticosteroids in severe IgAN and HSN have been shown by others [11–13]. The further decline in Ua/c after additional CYC-P therapy found in our study has also been shown in small series of high-risk patients [8–10, 15, 16]. On the other hand, CYC-P as single therapy was not shown to be more effective than supportive therapy [32]. In our study 50% of the patients treated with corticosteroids and CYC-P had a good outcome after a median 3.1 years, and no severe side effects were seen.
In our study neither the clinical features (mild or severe) nor the ISKDC grade of the biopsy (< III or ≥ III) could predict outcome, which has also been reported by other authors [2, 16, 27, 31, 33]. An early biopsy is, however, informative and of great importance for the further decision to treat. A high proportion of crescents in the first renal biopsy indicated treatment but did not predict poor outcome in our study. Instead, we found that a high percentage of SGS was more frequent among patients with poor outcome. Several investigators have reported that the number of crescents in the biopsy have a strong relation to outcome [15, 16, 34], while others have indicated that the predictive role of crescents is more controversial [1, 3, 35]. In our study, one patient who was anuric and had 67% of crescents in the first biopsy was treated as in group A and underwent peritoneal dialysis for 5 days. The patient recovered and 3 months later, he had normal GFR and blood pressure and only microalbuminuria. Four years after onset he is still healthy and takes no medication. In contrast, another patient who had nephrotic proteinuria, reduced GFR and a high percentage of SGS in the first biopsy, which were all resistant to intervention, went to ESRD within 6 months, despite aggressive treatment.
Fourteen percent of the patients progressed to ESRD, which is in agreement with other reports [15, 32, 33, 36]. Those patients had a lower GFR, greater proteinuria, and more severe morphological findings in their first renal biopsy, as has been reported in other studies [1, 4, 35, 37, 38].
In conclusion, we emphasize the value of an early biopsy in patients with severe clinical features. Treatment with MP and CYC-P in combination with ACEi/ARB, and treatment with corticosteroids in combination with ACEi/ARB, was efficient in reducing proteinuria and improving GFR in both HSN and IgAN patients. Although further studies are needed to determine the role of immunosuppression, our study shows that 50% of the patients undergoing the combined treatment had a good outcome at follow-up, despite severe clinical features and advanced renal biopsy findings.
References
D’Amico G (2004) Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Semin Nephrol 24:179–196
Coppo R, Mazzucco G, Caglioni L, Lupo A (1997) Long-term prognosis of Henoch-Schonlein nephritis in adults and children. Italian Group of Renal Immunopathology Collaborative Study on Henoch-Schonlein purpura. Nephrol Dial Transplant 12:2277–2283
Coppo R, Andrulli S, Amore A, Gianoglio B, Conti G, Peruzzi L, Locatelli F, Cagnoli L (2006) Predictors of outcome in Henoch-Schonlein nephritis in children and adults. Am J Kidney Dis 47:993–1003
Haas M (1997) Histologic subclassification of IgA nephropathy: a clinicopathologic study of 244 cases. Am J Kidney Dis 29:829–842
Wyatt RJ, Emancipator SN, Kon V, Waldo FB, Donadio J, Grande JP, Andreoli SP, Glassock RJ (1997) IgA nephropathy databank: development of a system for management of renal biopsy acquired data. Am J Kidney Dis 26:817–828
Andreoli SP, Bergstein JM (1989) Treatment of severe IgA nephropathy in children. Pediatr Nephrol 3:248–253
Choi MJ, Eustace JA, Gimenez LF, Atta MG, Scheel PJ, Sothinathan R, Briggs WA (2002) Mycophenolate mofetil treatment for primary glomerular diseases. Kidney Int 61:1098–1114
Flynn JT, Smoyer WE, Bunchman TE, Kershaw DB, Sedman AB (2001) Treatment of Henoch-Schonlein purpura glomerulonephritis in children with high-dose corticosteroids plus oral cyclophosphamide. Am J Nephrol 21:128–133
Iijima K, Ito-Kariya S, Nakamura H, Yoshikawa N (1998) Multiple combined therapy for severe Henoch-Schonlein nephritis in children. Pediatr Nephrol 12:244–248
Oner A, Tinaztepe K, Erdogan O (1995) The effect of triple therapy on rapidly progressive type of Henoch-Schonlein nephritis. Pediatr Nephrol 9:6–10
Niaudet P, Habib R (1998) Methylprednisolone pulse therapy in the treatment of severe forms of Schonlein-Henoch purpura nephritis. Pediatr Nephrol 12:238–243
Pozzi C, Andrulli S, Del Vecchio L, Melis P, Fogazzi GB, Altieri P, Ponticelli C, Locatelli F (2004) Corticosteroid effectiveness in IgA nephropathy: long-term results of a randomized, controlled trial. J Am Soc Nephrol 15:157–163
Pozzi C, Bolasco PG, Fogazzi GB, Andrulli S, Altieri P, Ponticelli C, Locatelli F (1999) Corticosteroids in IgA nephropathy: a randomised controlled trial. Lancet 353:883–887
Ronkainen J, Autio-Harmainen H, Nuutinen M (2003) Cyclosporin A for the treatment of severe Henoch-Schonlein glomerulonephritis. Pediatr Nephrol 18:1138–1142
Scharer K, Krmar R, Querfeld U, Ruder H, Waldherr R, Schaefer F (1999) Clinical outcome of Schonlein-Henoch purpura nephritis in children. Pediatr Nephrol 13:816–823
Shenoy M, Bradbury MG, Lewis MA, Webb NJ (2007) Outcome of Henoch-Schonlein purpura nephritis treated with long-term immunosuppression. Pediatr Nephrol 22:1717–1722
Shin JI, Park JM, Shin YH, Kim JH, Lee JS, Kim PK, Jeong HJ (2005) Can azathioprine and steroids alter the progression of severe Henoch-Schonlein nephritis in children? Pediatr Nephrol 20:1087–1092
Tanaka H, Suzuki K, Nakahata T, Ito E, Waga S (2003) Early treatment with oral immunosuppressants in severe proteinuric purpura nephritis. Pediatr Nephrol 18:347–350
Wyatt RJ, Hogg RJ (2001) Evidence-based assessment of treatment options for children with IgA nephropathies. Pediatr Nephrol 16:156–167
Yoshikawa N, Honda M, Iijima K, Awazu M, Hattori S, Nakanishi K, Ito H (2006) Steroid treatment for severe childhood IgA nephropathy: a randomized, controlled trial. Clin J Am Soc Nephrol 1:511–517
Yoshikawa N, Ito H, Sakai T, Takekoshi Y, Honda M, Awazu M, Ito K, Iitaka K, Koitabashi Y, Yamaoka K, Nakagawa K, Nakamura H, Matsuyama S, Seino Y, Takeda N, Hattori S, Ninomiya M (1999) A controlled trial of combined therapy for newly diagnosed severe childhood IgA nephropathy. The Japanese Pediatric IgA Nephropathy Treatment Study Group. J Am Soc Nephrol 10:101–109
Coppo R, Peruzzi L, Amore A, Piccoli A, Cochat P, Stone R, Kirschstein M, Linne T (2007) IgACE: a placebo-controlled, randomized trial of angiotensin-converting enzyme inhibitors in children and young people with IgA nephropathy and moderate proteinuria. J Am Soc Nephrol 18:1880–1888
Tanaka H, Suzuki K, Nakahata T, Tsugawa K, Konno Y, Tsuruga K, Ito E, Waga S (2004) Combined therapy of enalapril and losartan attenuates histologic progression in immunoglobulin A nephropathy. Pediatr Int 46:576–579
Meadow SR, Glasgow EF, White RH, Moncrieff MW, Cameron JS, Ogg CS (1972) Schonlein-Henoch nephritis. Q J Med 41:241–58
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (2004) The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114:555–576
Berg U, Bohlin AB (1982) Renal hemodynamics in minimal change nephrotic syndrome in childhood. Int J Pediatr Nephrol 3:187–192
Counahan R, Winterborn MH, White RH, Heaton JM, Meadow SR, Bluett NH, Swetschin H, Cameron JS, Chantler C (1977) Prognosis of Henoch-Schonlein nephritis in children. BMJ 2:11–14
Foster BJ, Bernard C, Drummond KN, Sharma AK (2000) Effective therapy for severe Henoch-Schonlein purpura nephritis with prednisone and azathioprine: a clinical and histopathologic study. J Pediatr 136:370–375
D’Amico G (2000) Natural history of idiopathic IgA nephropathy: role of clinical and histological prognostic factors. Am J Kidney Dis 36:227–237
Pillebout E, Thervet E, Hill G, Alberti C, Vanhille P, Nochy D (2002) Henoch-Schonlein purpura in adults: outcome and prognostic factors. J Am Soc Nephrol 13:1271–1278
Ronkainen J, Ala-Houhala M, Huttunen NP, Jahnukainen T, Koskimies O, Ormala T, Nuutinen M (2003) Outcome of Henoch-Schoenlein nephritis with nephrotic-range proteinuria. Clin Nephrol 60:80–84
Tarshish P, Bernstein J, Edelmann CM Jr (2004) Henoch-Schonlein purpura nephritis: course of disease and efficacy of cyclophosphamide. Pediatr Nephrol 19:51–56
Goldstein AR, Bernstein J, Edelmann CM Jr (1992) Long-term follow-up of childhood Henoch-Schonlein nephritis. Lancet 339:280–282
Davin JC, Weening JJ (2001) Henoch-Schonlein purpura nephritis: an update. Eur J Pediatr 160:689–695
Coppo R, D’Amico G (2005) Factors predicting progression of IgA nephropathies. J Nephrol 18:503–512
Ronkainen J, Nuutinen M, Koskimies O (2002) The adult kidney 24 years after childhood Henoch-Schonlein purpura: a retrospective cohort study. Lancet 360:666–670
Hogg RJ, Silva FG, Wyatt RJ, Reisch JS, Argyle JC, Savino DA (1994) Prognostic indicators in children with IgA nephropathy—report of the Southwest Pediatric Nephrology Study Group. Pediatr Nephrol 8:15–20
Yoshikawa N, Ito H, Nakamura H (1992) Prognostic indicators in childhood IgA nephropathy. Nephron 60:60–67
Acknowledgments
This study was financially supported by the Samariten Foundation, the Frimurarna Association, the Jerring Foundation and the Swedish Medical Research Council (no. 6864). Financial support was also provided through the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and the Karolinska Institutet.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Edström Halling, S., Söderberg, M.P. & Berg, U.B. Treatment of severe Henoch–Schönlein and immunoglobulin A nephritis. A single center experience. Pediatr Nephrol 24, 91–97 (2009). https://doi.org/10.1007/s00467-008-0990-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-008-0990-z