Abstract
To explore the potential protective role of urogenital lactobacilli against urinary tract infection (UTI), lactobacillus cultures were performed on stool and urine specimens and periurethral/vaginal swabs of febrile infants who were suspected of having UTI. Those infants diagnosed with UTI based on the results of the suprapubic urine cultures were allocated to the UTI group (n = 60), and those who had a simple viral illness with negative urine cultures were allocated to the control group (n = 31). Lactobacilli were anaerobically cultured in lactobacillus-specific DifcoTM Rogosa SL agar for 48 h at 37°C and then counted. The lactobacillus colony counts for the stool and urine specimens and periurethral swabs from the UTI group were significantly lower than those for the control group (P < 0.05). The geometric means of stool, periurethra, and urine lactobacilli in the UTI group were significantly lower than those in the control group (P < 0.05). The colony count of the vaginal lactobacillus demonstrated an equivocal difference between the UTI and control group. In conclusion, this is the first prospective case–control study to demonstrate reduced lactobacillus urogenital colonization in infants with UTI. Our results support the view that less urogenital lactobacillus colonization may be a risk factor for UTI in infants even though there is an unclear possibility that the UTI itself could be the cause of the lower lactobacillus colonies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary tract infection (UTI) is known as an ascending type of infection by fecal uropathogens, as documented by a genomic profile study [1]. The imbalance between uropathogens and normal flora has been suggested to be a risk factor for UTI [2].
A 1915 study reported that cystitis could be successfully treated by intravesical injection of a lactobacillus strain [3], but this finding was ignored during the following era of widespread antibiotic use. Subsequent evidence on the role of lactobacilli against urogenital infection came from a 1973 study which showed significantly depleted vaginal lactobacilli in women with recurrent urethritis relative to healthy women [4]. An inverse association between hydrogen peroxide (H2O2)-producing lactobacilli and Escherichia coli colonization was demonstrated in the vaginal discharge of the women with recurrent UTI [5]. The inhibitory effects of vaginal lactobacilli against E. coli has also been recognized [6]. Aerobic and anaerobic urethral flora of healthy women were found to be quite different from those of UTI women [7]. Intraurethrally administered indigenous lactobacilli were also found to prevent the development of UTI ostensibly in animal models [6, 8–10]. Human breast milk, which was demonstrated to be a natural source of lactobacilli, was found to have a protective effect against UTI in infants [11, 12]. Several clinical trials in adults reported that a number of well-characterized lactobacillus strains improved vaginal ecology and prevented urogenital infection [13–15]. Among the pediatric population, there has been one case report of successful lactobacillus prophylaxis in a girl with recurrent UTI [16], and there has been a recent prospective study in which oral lactobacillus prophylaxis was effective in preventing persistent primary vesicoureteral reflux (VUR) [17]. However, to date, there has been no information published on the lactobacillus status of children.
The object of this study was to evaluate the genitourinary and fecal lactobacillus status of infants with UTI.
Subjects and methods
Three specimens (stool, urine, and periurethral/vaginal swab) were obtained for lactobacillus culture from 91 febrile infants admitted to Ewha Womans University Medical Center between March 2005 and March 2006. Febrile infants with a past history of UTI and antibiotic treatment before the time of the urine sample were excluded from the study. Inclusion criteria of the UTI group were infants with unexplained fever who were suspected of having UTI. The exclusion criteria were genitourinary tract anomaly/malformation and obstructive uropathy. The UTIs were confirmed by significant bacteriuria on the suprapubic aspirated urine culture (single uropathogen >103 CFU/mL). During the same period, control samples were obtained in infants who were suspected of having simple viral illness [acute bronchiolitis (18 infants), exanthem subitum (three infants), upper respiratory infection (ten infants)]. Each sample for lactobacillus was adequately prepared for anaerobic culture. All UTI-suspicious infants were initially treated with ceftriaxone after three samples had been taken for lactobacillus culture. Urine cultures were collected by suprapubic aspiration in febrile infants who were suspected of having UTI and by sterile bag urine culture in control infants. The imaging studies [renal ultrasound; technetium-99m dimercatosuccinic acid scintigraphy (99mTC DMSA) and voiding cystourethrography (VCUG)] were performed on infants with definite UTI. Of the infants with UTI, 41.6% (25/60) had 99mTC DMSA (+) pyelonephritis and 18.3% (11/60) had VUR.
Lactobacillus was anaerobically cultured in lactobacillus-selective DifcoTM Rogosa agar (Becton, Dickinson and Co, Franklin Lakes, NJ) at 37°C for 48 h, at which time the colonies were counted and the colony forming units (CFU)/mL determined. The study protocol was approved by the ethical committee of the hospital and informed consent was obtained from each parent. The calculated sample size to detect a 50% difference between the two groups at a value of 0.05 (one-sided) and a power of 0.80 was 91 cases.
When 100 samples of lactobacillus were obtained, the study was terminated. Infants (n = 3) with negative urine cultures or less than significant bacteriuria (single uropathogen <103 CFU/mL) and infants (n = 6) with positive urine culture in the control group were excluded from further study. A total of 60 infants were ultimately allocated to the UTI group and 31 infants to the control group. The age (5.6 ± 3.9 vs. 5.3 ± 3.4 months, respectively), male-to-female ratio (41:19 vs. 25:6, respectively), duration of fever, and feeding type were not significantly different between the two groups (Table 1).
For statistical analysis, the Mantel-Haenszel κ2 was used to compare the distribution of lactobacillus CFU from the stool and urine specimens and the periurethral/vaginal swabs. The Spearman correlation and Wilcoxon rank sum test were used to compare the CFUs of lactobacilli between the two groups. All data were expressed as mean ± standard deviation. A P value <0.05 was considered to be statistically significant.
Results
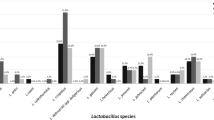
Lactobacillus colonies in the control group were most abundant in the stool cultures, less abundant in the periurethra/vagina cultures, and least abundant in the urine cultures. The numbers of stool lactobacilli were over 103 CFU/mL in all infants, whereas there was less than 103 CFU/mL in the majority of infants (Fig. 1).
There were significantly lower levels of lactobacilli colonies in the urine cultures of the UTI group than in those of the control group (P < 0.05). In the UTI group, 95.0% (57/60) of the infants had less than 103 CFU/mL of urine lactobacilli, which was significantly higher than the 67.7% (21/31) in the control group (P < 0.01). Among the male infants, there were significantly lower levels of periurethral lactobacilli colonies in the UTI group than in the control group (P < 0.01). In the UTI group, 43.9% (18/41) of the infants had less than 103 CFU/mL of periurethral lactobacilli, which was significantly higher than the 8% (2/25) in the control group (P < 0.01). Among the female infants, the level of vaginal lactobacilli colonies in the UTI group was not significantly different from those of the control group (P = 0.074), but 26.4% (5/19) of the UTI group had less than 103 CFU/mL of vaginal lactobacilli, which was significantly higher than the 0% (0/6) in the control group (P = 0.03). There were also significantly lower levels of lactobacillus colonies in the stool cultures of the UTI group than in those in the control group (P < 0.01). In the UTI group, 11.6% (7/60) had less than 103 CFU/mL of stool lactobacilli, which was significantly higher than the 0% (0/31) in the control group (P < 0.01) (Table 2). In the UTI group, we analyzed the number of each lactobacilli colony according to fever duration, urine collection time, and the response time to antibiotics; none of these parameters were significant between the two groups.
The geometric means of lactobacillus CFUs in the UTI group were significantly lower than those of the control group in the stool cultures (19,286 ± 30,920 vs. 16,969,129 ± 89,717,956, respectively) (P < 0.01), the male periurethras (2,814 ± 5,672 vs. 12,448 ± 25,443) (P < 0.05), and the urine cultures (173 ± 469 vs. 2,087 ± 5,681) (P < 0.05). However, the geometric mean of vaginal lactobacilli in the UTI group was not significantly different from that of the control group (2,731 ± 4,781 vs. 5,500 ± 4,394) (P = 0.16) (Fig. 1). This statistical insignificance may be due to the small number of cases.
Discussion
In this first prospective study, there were clearly fewer lactobacillus colonies in the stool, periurethra, and urine cultures and a not as distinct lower number in the vagina swabs of UTI infants than in those of the control infants. In adults, a significant depletion of vaginal lactobacilli in women with recurrent urethritis has been reported: vaginal lactobacilli were cultured only in 61% of women with recurrent urethritis, compared with 97% of healthy control women [4]. A more recent study also showed an inverse association between H2O2-producing lactobacilli and E. coli colonization in the vagina of women with recurrent UTI. The women without H2O2-producing lactobacilli were more likely to have introital E. coli colonization than those with H2O2-producing lactobacilli (Odds ratio 4.0; 95% confidence interval 1.3–11.6; P = 0.01). The absence of H2O2-producing lactobacilli has been suggested to be an important factor in the pathogenesis of recurrent UTI [5]. That the intravaginal administration of indigenous lactobacilli can eliminate vaginal E. coli colonization and inhibit E. coli has long been recognized [6]. In a study of urethral flora (first 10 mL of voided urine), lactobacilli were also dominant organisms, accounting for 14.6–28.3% of the total aerobic and anaerobic flora. Lactobacilli were cultured in 80% of healthy reproductive-age women compared with 50% of UTI women [7]. These results differ from our data, which showed that vaginal and periurethral lactobacilli were cultured in 94.8% of UTI infants and 87.8% of male UTI infants, respectively; these values are lower but not significantly different to the 100% figure in control infants. This discrepancy may be related to the difference in the study population (infant with first UTI in our study vs. women with recurrent urethritis or UTI) and a small sample size.
There are several mechanisms through which lactobacilli prevent the colonization of uropathogens. In in vitro tests, lactobacilli were proven to adhere to the mucus and form a biosurfactant barrier that disrupts the adherence of uropathogens to the uroepithelial receptors [18, 19] and produces antimicrobial compounds, such as H2O2, lactic acid, and bacteriocin, that in turn interferes with the growth of uropathogens [20]. It has also been proven that lactobacilli modulates the host immune system by stimulating dendritic cell, T-cell, and phagocytic activity, by up-regulating Th-2 anti-inflammatory cytokines [interleukin (IL)-10, IL-12] and proinflammatory cytokines (IL-6), and by down-regulating inflammatory cytokines (IL-8, cyclooxygenase) or virulence factor expressions [21, 22].
In an animal model, indigenous L. casei strains that were instilled into the bladders before challenge with uropathogens prevented UTIs in 84% of the tested rats [8], and vaginal flush with indigenous lactobacilli eliminated vaginal E. coli colonization in four adult monkeys with pyelonephritogenic E. coli infection [6]. A single intraurethral administration of L. casei Shirota strain before the challenge of E. coli dramatically inhibited E. coli growth and the subsequent inflammatory response in the mouse urinary tracts [9].
In human clinical trials, the intravaginal instillation of L. rhamnosus GR-1 and L. fermentum RC-14 stimulated indigenous vaginal lactobacilli and reduced the UTI recurrence rate from 6 to 1.6 episodes per year in women [13, 14] and was also effective in reducing the recurrence rate of UTI in 41 UTI women—from 47 to 21%—even after antimicrobial therapy [15]. In premature infants, breast milk supplemented with L. rhamnosus GG reduced the number of UTI episodes, although the difference was not statistically significant [23]. In children, there has been a single case report showing successful probiotic prophylaxis in a 6-year-old girl with frequently recurrent UTI, who was given L. acidophilus DDS-1 (2 × 109 CFU) twice daily for a month and then once daily for 5 months; she had no recurrent UTI, which is in contrast to three consecutive relapses contracted for the preceding 3 months [16]. The role of oral probiotics in the resolution of the urogenital infection including UTI has been the subject of recent reports [24, 25]. The importance of urogenital probiotics could be accentuated by the avoidance of long-term use of antibiotics and the subsequent emergence of resistant strains [26]. Another advantage would be that it is the natural approach that replenishes the depleted normal flora to create a better environment to fight off uropathogens. There are still many questions to be resolved, but promising evidence on urogenital lactobacilli suggest the possible benefits and may provide the rationale for studying lactobacilli as prophylaxis to prevent UTI, even in children. In order to develop the ideal urogenital probiotics, it is important to identify the best lactobacillus strains that are detrimental to uropathogens in further studies [27].
In conclusion, this is the first prospective case–control study to demonstrate reduced lactobacillus urogenital colonization in infants with UTI. Our results supports the view that lower urogenital lactobacillus colonization may be a risk factor for UTI in infants even though there is an unclear possibility that the UTI itself may actually have caused the low lactobacillus colony count. The limitation of our study is in not being able to clarify whether the difference in the number of lactobacilli colonies originated simply from the difference in bacterial or viral infections. Further clinical studies are needed to clarify the effect of lactobacilli in preventing UTI in infants.
References
Usein CR, Damian M, Tatu-Chitoiu D, Capusa C, Fagara R, Mircescu G (2003) Comparison of genomic profiles of Escherichia coli isolates from urinary tract infections. Roum Arch Microbiol Immunol 62:137–154
Stamey TA, Sexton CC (1975) The role of vaginal colonization with Enterobacteriaceae in recurrent urinary tract infections. J Urol 113:214–217
Newman D (1915) The treatment of cystitis by intravesical injection of lactic bacillus cultures. Lancet 14:330–332
Bruce AW, Chadwick P, Hassan A, van Cott GF (1973) Recurrent urethritis in women. Can Med Assoc J 108:973–976
Gupta K, Stapleton AE, Hooton TM, Roberts PL, Fennell CL, Stamm WE (1998) Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli colonization in woman with recurrent urinary tract infections. J Infect Dis 178:446–450
Herthelius M, Gorbach SL, Mollby R, Nord CE, Pettersson L, Winberg J (1989) Elimination of vaginal colonization with Escherichia coli by administration of indigenous flora. Infect Immun 57:2447–2251
Marie TJ, Swantee CA, Hartlen M (1980) Aerobic and anaerobic uretheral flora of healthy females in various physiologic age groups and of females with urinary tract infections. J Clin Microbiol 11:654–659
Reid G, Chan RC, Bruce AW, Costerton JW (1985) Prevention of urinary tract infection in rats with indigenous Lactobacillus casei strain. Infect Immun 49:320–324
Asahara T, Nomoto K, Watanuki M, Yokokura T (2001) Antimicrobial activity of intraurethrally administered probiotic Lactobacillus casei in a murine model of Escherichia coli urinary tract infection. Antimicrob Agents Chemother 45:1751–1760
Fraga M, Scavone P, Zunino P (2005) Preventive and therapeutic administration of an indigenous lactobacillus sp. strain against Proteus mirabilis ascending urinary tract infection in a mouse model. Antonie van Leeuwenhoek 88:25–34
Martin R, Langa S, Reviriego C, Jiminez E, Marin ML, Xaus J, Fernández L, Rodríguez JM (2003) Human milk is a source of lactic acid bacteria for the infant gut. J Pediatr 143:754–758
Marild S, Hansson S, Jodal U, Oden A, Svedberg K (2004) Protective effect of breastfeeding against urinary tract infection. Acta Pediatr 93:164–168
Bruce AW, Reid G (1988) Intravaginal instillation of lactobacilli for prevention of recurrent urinary tract infection. Can J Microbiol 34:339–343
Reid G, Bruce AW, Taylor M (1995) Instillation of Lactobacillus and stimulation of indigenous organisms to prevent recurrence of urinary tract infection. Microecol Ther 23:32–45
Reid G, Bruce AW, Taylor M (1992) Influence of three-day antimicrobial therapy and lactobacillus vaginal suppositories on recurrence of urinary tract infection. Clin Ther 14:11–16
Gerasimov SV (2004) Probiotic prophylaxis in pediatric recurrent urinary tract infection. Clin Pediatr 43:95–98
Lee SJ, Shim YH, Cho SJ, Lee JW (2007) Probiotics prophylaxis in children with persistent primary vesicoureteral reflux. Pediatr Nephrol 22:1315–1320
Chan RC, Bruce AW, Reid G (1984) Adherence of cervical, vaginal and distal urethral normal microbial flora to human uroepithelial cells and the inhibition of adherence of gram-negative uropathogens by competitive exclusion. J Urol 131:596–601
Velraeds MC, van der Mei HC, Reid G, Busscher HJ (1996) Inhibition of initial adhesion of uropathogenic Enterococcus faecalis by biosurfactant from lactobacillus isolates. Appl Environ Microbiol 62:1958–1963
Osset J, Bartolome RM, Garcia E, Andreu A (2001) Assessment of the capacity of lactobacillus to inhibit the growth of uropathogens and block their adhesion to vagina epithelial cells. J Infect Dis 183:485–491
Gill HS, Rutherfurd KJ, Prasad J, Gopal PK (2000) Enhancement of natural and acquired immunity by Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HN019). Br J Nutr 83:167–176
Matsuzaki T, Chin J (2000) Modulating immune responses with probiotic bacteria. Immunol Cell Biol 78:67–73
Dani C, Biadaioli R, Bertini G, Martelli E, Rubaltelli FF (2002) Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. A prospective double-blind study. Biol Neonate 82:103–108
Reid G, Bruce AW, Fraser N, Heinemann C, Owen J, Henning B (2001) Oral probiotics can resolve urogenital infection. FEMS Immunol Microbiol 30:49–52
Reid G, Bruce AW (2006) Probiotics to prevent urinary tract infections: the rationale and evidence. World J Urol 24:28–32
Food and Agriculture Organization (FAO) (2001) Evaluation of health and nutritional properties of probiotics in food including powder milk and live lactic acid bacteria. Food and Agriculture Organization of the United States and World Health Organization Expert Consultation Report. Available at https://doi.org/www.fao.org/es/ESN/Probio/probio.html2001. Cordoban, Argentina
Reid G, Bruce AW (2001) Selection of lactobacillus strains for urogenital probiotic applications. J Infect Dis 183:S77–S80
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, J.W., Shim, Y.H. & Lee, S.J. Lactobacillus colonization status in infants with urinary tract infection. Pediatr Nephrol 24, 135–139 (2009). https://doi.org/10.1007/s00467-008-0974-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-008-0974-z