Abstract
The angiopoietin/Tie-2 system plays an important role in the initiation of angiogenesis. However, the role of angiopoietin/Tie-2 in peritoneal angiogenesis and fibrosis is unclear. In our study we investigated the peritoneal morphologic changes in a uremic peritoneal dialysis (PD) rat model, focusing on the relationship between angiopoietin/Tie-2 and peritoneal angiogenesis. We subjected uremic (subtotal nephrectomy) rats to dialysis, using a standard PD solution, for 10 days, 28 days, or 56 days, and compared them with uremic rats that had not undergone dialysis and control rats. Functional [dialysate-to-plasma (D/P) creatinine; ultrafiltration (UF)] and structural (vessel density and thickness of the submesothelial extracellular matrix) changes of the peritoneum were quantified. Levels of angiopoietin (Ang)-1, Ang-2, Tie-2 and vascular endothelial growth factor (VEGF) were examined in the peritoneum by real-time quantitative polymerase chain reaction (PCR) and related to angiogenesis. The uremic group that had not undergone dialysis was characterized by increased vessel density in the peritoneum compared with that of the control, which correlated with decreased UF and increased D/P creatinine. Progressive angiogenesis and fibrosis were found in the PD groups when compared with the uremic non-dialyzed or control group, accompanied by an increased D/P creatinine that occurred in the PD group after 56 days, while UF decreased. Furthermore, Ang-2 and VEGF levels increased, while Tie-2 level decreased significantly in the uremic non-dialyzed group compare with the control. This tendency was more obvious in the PD groups than in the uremic non-dialyzed or control group, but no difference was found among the PD groups. Both VEGF and Ang-2 correlated positively with vessel density, while Tie-2 correlated negatively. We confirmed angiogenesis and fibrosis changes of the peritoneum as a result of uremic status and PD therapy in the uremic PD rat model. An increased level of Ang-2 and a reduced level of Tie-2 in conditions of uremia and PD therapy correlated with peritoneal angiogenesis and functional deterioration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ultrafiltration failure in peritoneal dialysis (PD) has been linked to neo-angiogenesis and fibrosis in the peritoneal membrane and is associated with therapy failure [1–3]. However, the signaling cascade mediating morphological changes to the peritoneum are incompletely understood.
Angiopoietin/Tie-2 is a recently identified endothelial cell-specific ligand/receptor system which plays an important role in the initiation of neo-angiogenesis [4, 5]. However, the role of angiopoietin/Tie-2 in peritoneal angiogenesis has not been studied. We hypothesized that this signaling system would reflect neo-angiogenesis in the peritoneum, and we investigated this hypothesis in uremic PD rats that were subjected to dialysis for 10–56 days.
Materials and methods
Animals
Thirty-five male Wistar rats (Chinese Academy of Science, Shanghai, China), weighing 180 ± 10 g, were studied under conventional laboratory conditions, with free access to food and water. The rats were randomly assigned to be in a control group (n = 6), a uremic group not subjected to PD (n = 7), or a uremic group subjected to PD (n = 22). The group of uremic rats undergoing PD were subdivided into three groups according to different durations of PD: 10 days (n = 6); 28 days (n = 6); 56 days (n = 6). The uremia was generated by 5/6 nephrectomy. The amount of renal tissue removed (approximately 85%) was weighed. Rats undergoing sham-operation (control) were subjected to bilateral flank incisions. Five weeks after the subtotal nephrectomy, a peritoneal catheter connected to a subcutaneous mini-vascular access port was implanted into each rat, which had been anesthetized with chloroaldehyde, 10% hydration. Dialysis was performed with 4.25% Dianeal (Baxter) 3 ml/100 g body weight once a day 1 week after implantation.
Peritoneal equilibration test
A peritoneal equilibration test (PET) was performed, then the rats were killed at three time points: after 10 days of PD, after 28 days of PD, and after 56 days of PD. The control group and the uremic non-dialyzed group underwent a PET and were killed after 10 days of PD. Each animal was injected with 21 ml of 2.5% Dianeal, then 1 ml of dialysate was taken for a glucose test immediately after infusion. After injection, the rats were allowed free access to water. Two hours later, the rats were anesthetized with chloroaldehyde, 10% hydration. The remaining dialysate was then completely drained and measured. At the same time, blood samples were taken for plasma analysis. The peritoneal transport characteristic was determined from the dialysate-to-plasma (D/P) ratios of creatinine. To exclude peritonitis, we cultured the peritoneal effluents for the presence of bacteria.
Morphologic examination of the omentum and parietal peritoneum
Omentum was taken after the rats had been killed and fixed in a sufficient amount of 4% phosphate-buffered formaldehyde for 24 h. The tissue samples were then embedded in paraffin and 5 μm sections were cut. The cut sections were stained for immunohistochemical examination carried out with antibody to CD31-related antigen (Dako Corporation, Carpenteria, CA, USA). The microscopic image was fixed at the position where there were most vessels with ×100 magnification. The vessels were counted in ten random fields.
Specimens of the parietal peritoneum were cut (8 μm) and stained with van Gieson stain (Shanghai Outdo Biotech Co., Shanghai, China); all biopsies were similarly embedded, sectioned, and stained. The thickness of the submesothelial extracellular matrix layer beneath the mesothelium was determined (in micrometers), and the average of ten independent measurements was calculated for each section (observed at ×400 magnification).
Real-time quantitative polymerase chain reaction
Angiopoietin (Ang)-1, Ang-2, Tie-2 and vascular endothelial growth factor (VEGF) (omentum) mRNA was analyzed by real-time SYBR Green polymerase chain reaction (PCR). Total RNA was isolated using Triazol reagent (Invitrogen, USA) according to the manufacturer’s recommendations. One microgram of the RNA was reverse transcribed to cDNA using random primer (Life Technologies BRL, Grand Island, NY, USA) with Maloney murine leukemia virus (MMLV) reverse transcriptase. Real-time PCR was done with Applied Biosystem TaqMan 7000 (Applera GmbH, Germany) in 384-well plates, using SYBR Green Master Mix (TaRaKa, Kyoto, Japan) in 5 μl reaction mixtures. The PCR mixture contained SYBR Green Master Mix 2.5 μl, primers forward and reverse 0.1 μl respectively, rox 0.1 μl, H2O 1.7 μl, cDNA 0.5 μl. The amplification program consisted of one cycle at 95°C with a 10 min hold, followed by 40 cycles (95°C, 15 s → 55°C, 30s → 72°C, 30 s). The oligonucleotide primer sequences were as follows: Ang-1 was 5′-CTCTATGCCAACACAGTGCT-3′ and 5′-GATCTTGATCTTCATGGTGCT-3′. Ang-2 was 5′-CGGCCACAGTCAACAACTCA-3′ and 5′-GCTCTTATAGTCGGGCGATGA-3′. Tie-2 was 5′-TGCCACCATCACTCAATACCA-3′ and 5′-AGGCTGGGTTGCTTGATCCT-3′. VEGF was 5′-TCCTGGAGCGTTCACTGTGA-3′ and 5′-CGCGAGTCTGTGTTTTTGCA-3′. β-actin served as an internal control. Primers for β-actin were 5′-AGGCCAACCGTGAAAAGATG-3′ and 5′-ACCAGAGGCATACAGGGACAA-3′. All full-length amplified fragments were 100 bp long. Relative gene copy number was calculated from the formula: \(2^{{\Delta \Delta Ct}} {\left( {\Delta \Delta {\text{CT}} = {\left( {{\text{CT}}_{{{\text{target}}}} - {\text{CT}}_{{{\text{ $ \beta $ }} - {\text{actin}}}} } \right)} - {\left( {{\text{CT}}_{{{\text{reference}}}} - {\text{CT}}_{{{\text{ $ \beta $ }} - {\text{actin}}}} } \right)}} \right)}\).
Statistical analysis
Data were expressed as means ± standard deviations (SDs) or medians and 25th to 75th interquartile ranges. Means were initially subjected to one-way analysis of variance (ANOVA), followed by multiple comparisons using the Dunnett test. Medians were analyzed statistically with the non-parametric Mann–Whitney U test. The correlations between Ang-2, Tie-2, VEGF and vessel density were analyzed by Pearson’s correlation test. In this study, statistical analysis was performed with SAS 6.12 software (USA) so that we could observe the significant difference. P values less than 0.05 were considered significant.
Results
Clinical, biological, and functional parameters
The body weight and plasma creatinine level of the rats are shown in Table 1. Weight decreased in the uremic non-dialyzed rats compared with control rats (P < 0.05), but no difference was found between the control and the PD 10 days group. The rats in the PD 28 days and PD 56 days groups had been killed at different times from those in the above groups, so no weight comparison was available among them. The uremic rats were characterized by plasma creatinine levels that were two- to three-times higher, 6 weeks after the subtotal nephrectomy. In our study, two rats were removed, due to unacceptable plasma creatinine levels, and three rats were removed because of peritonitis.
Functional parameters of the peritoneum evaluated when the rats were killed are shown in Table 2. Net ultrafiltration (UF) decreased in the uremic non-dialyzed group compared with the control group and further decreased in the PD 56 days group. The transport rate of small solutes (i.e. D/P creatinine) increased in the uremic non-dialyzed group compared with the control and further increased in the PD 56 days group.
Blood vessel density in omentum
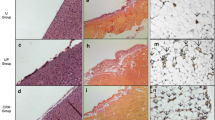
We counted the number of vessel in the peritoneal tissue after CD31 staining. Vessel density in the uremic non-dialyzed group [5 ± 3 per high-power field (HP)] increased in comparison with that in the control group (1 ± 1/HP) (P < 0.05). Peritoneal dialysis treatment markedly augmented vessel density in the dialysis groups compared with the control and uremic non-dialyzed groups (P < 0.05). In the dialysis groups, vessel density significantly increased from the PD 10 days group (10 ± 5/HP) to the 28 days group (17 ± 5/HP) (P < 0.05), but no significant difference in vessel density between the PD 28 days group and the 56 days group (19 ± 4) was found. The data are presented as the means and SDs in a bar graph (Fig. 1).
a Representative field showing the different omental vessel densities in the rats after immunohistochemistry staining with anti-CD31 (×100). b The number of blood vessels per field after anti-CD31 staining, assessed in the omentum under ×100 magnification. * P < 0.05 vs control rats; # P < 0.05 vs uremic rats. Scale bars represent 20 μm
Thickness of the submesothelial extracellular matrix in parietal peritoneum
Matrix thickness of the peritoneum demonstrated no difference between the control group (10.3 ± 5 μm) and the uremic non-dialyzed group (13.2 ± 3.8 μm). However, it had significantly increased in the dialysis groups (P < 0.05). Different durations of dialysis had resulted in increasing thickness of submesothelial extracellular matrix (27.3 ± 9.8 μm, 43 ± 17 μm and 63.5 ± 16.3 μm, respectively) (P < 0.05) (Fig. 2).
a Representative fields showing the thickness of van Gieson-stained submesothelial extracellular matrix in parietal rat peritoneum at ×100 magnification. b Measured thickness of van Gieson-stained submesothelial extracellular matrix in parietal peritoneum (×100 magnification). * P < 0.05 vs control rats; # P < 0.05 vs uremic rats. Scale bars represent 50 μm
Molecular biologic analysis
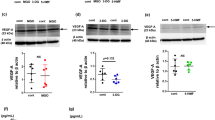
All samples tested showed no primer-dimers in the melting curve analysis. The level of Ang-2 was higher in the uremic non-dialyzed group than in the control group and was markedly enhanced by PD. Significant correlation existed between the level of Ang-2 and omental vessel density (r = 0.7756, P < 0.001) (Fig. 3). No difference in level of Ang-1 was found among the groups. The Tie-2 level in the uremic non-dialyzed group was less than that of the control group and decreased further in the dialysis groups. However, no significant difference in Tie-2 level was observed among the PD groups. There was a negative correlation between Tie-2 level and omental vessel density (r = −0.6433, P < 0.001) (Fig. 4). VEGF level also increased significantly in the uremic non-dialyzed group compared with the control group. In the PD group, the level of VEGF further increased in the PD 10 days and PD 28 days groups, but no difference in level of VEGF was found between the PD 56 days group and the uremic non-dialyzed group. A positive correlation also existed between the level of VEGF and omental vessel density (r = 0.5223, P < 0.05) (Fig. 5).
Discussion
In our study we confirmed angiogenesis and fibrosis changes of the peritoneal membrane as a result of uremic status and PD therapy. Structural changes may contribute to ultrafiltration failure of the peritoneal membrane. However, the relationship between peritoneal fibrosis and angiogenesis has not been clearly defined. Studies in PD patients have found that vasculopathy predisposes patients to the development of fibrosis and increased blood vessel density in the submesothelial zone in those with peritoneal ‘sclerosis’, compared with control subjects [6, 7]. Others have also shown that anti-angiogenic treatment inhibited intra-abdominal adhesion formation and was able to prevent peritoneal fibrosis in encapsulated peritoneal sclerosis [8, 9]. Furthermore, Margetts and colleagues used adenovirus (Ad)-mediated gene transfer of angiostatin, a recognized angiogenesis inhibitor, and decorin, a transforming growth factor-beta-inhibiting proteoglycan, in a daily infusion model of peritoneal dialysis, to identify the relative importance of peritoneal fibrosis and angiogenesis in peritoneal membrane dysfunction. They found that animals treated with AdAngiostatin demonstrated an improvement in net ultrafiltration with a significant reduction in vessel density in comparison with an AdDecorin group. Therefore, they drew the conclusion that peritoneal angiogenesis is relatively more important than fibrosis in peritoneal membrane dysfunction [10]. On the other hand, fibrosis is the final common pathway for nearly all forms of disease that progress towards end-stage organ failure [11]. In our study, peritoneal vessel density in the uremic non-dialyzed group increased significantly in comparison with that in the control group, but no difference in matrix thickness was found between them. Furthermore, in comparison with that in the PD 28 days group, the vessel density in the PD 56 days group had not increased significantly, while fibrosis was more obvious. Our study confirmed that neo-angiogenesis was the early pathological change of the peritoneum and that fibrosis of the interstitium following neo-angiogenesis contributed to the permanent loss of peritoneal tissue structure and function failure. It will be more convincible to elucidate the relationship between fibrosis and angiogenesis of the peritoneum if we examine the different expression times and relationships of pro-angiogenic and pro-fibrogenic growth factors, for example, transforming growth factor beta (TGF-β), or tissue inhibitor of matrix metalloproteinase (TIMP)-1, in the future.
Bio-incompatible PD fluids and uremic status are considered to be the main etiologic factors that lead to structural and functional changes of the peritoneum. To date, however, most experimental PD models have used non-uremic animals and thus underestimate the PD fluid-induced injury under uremic conditions. We analyzed peritoneal morphologic changes secondary to uremia and a combination of both conditions at two levels, better simulating the pathophysiological process of PD. First, at the time point of PD 10 days, we compared the sham operation, uremic non-dialyzed and uremia with PD 10 days groups. Second, different PD durations in uremic rats were observed. It was a limitation of the study that we did not have a matching control group and uremic non-dialyzed group at PD 28 days and PD 56 days, respectively. However, in a previous experiment, we set up matching groups with sham operation without PD at different time point; however, we found no structural difference between the sham operation rats when killed at different times. The Biopsy Registry data on humans had shown that increased thickness of parietal peritoneum preceded PD therapy, i.e. it was present as the result of uremia both before dialysis and in patients treated only with hemodialysis (HD) [12]. Inconsistent with those authors’ findings, our data on morphological changes of the peritoneum showed that rapid development of fibrosis was induced by exposure to PD solutions rather than by uremia itself. The uremic non-dialyzed rats were killed only 10 days after uremia had appeared, and a limited number of animals was investigated in our study. This might partially explain why fibrosis was not significant in the uremic non-dialyzed rats observed in our study. Clearly, such results should be treated with caution, and a long-term study with larger sample size is needed.
Angiopoietin and its Tie-2 receptor constitute another recently identified endothelial cell-specific, ligand–receptor system that is crucial in vascular development. In this study, we focused on the relationship between angiopoietin/Tie-2 and angiogenesis of the peritoneum. Of the four currently known angiopoietin family members, the best characterized ones are Ang-1 and Ang-2, both of which function as ligands for the endothelium-specific tyrosine kinase receptor, Tie-2 [13, 14]. Ang-1, expressed primarily by perivascular cells, is an activator of Tie-2 and is required for blood vessel stabilization and maturation during development [15, 16]. Ang-2, which is thought to be an endogenous antagonist of Ang-1, competes for binding to the Tie-2 receptor and blocks the Ang-1-induced Tie-2 autophosphorylation during vasculogenesis, subsequently leading to loosening of cell–matrix and cell–cell contacts and allowing access to angiogenesis inducers. Ang-2 is selectively expressed in the endothelial cells of actively remodeling vessels [17]. In the presence of VEGF, vessel destabilization caused by Ang-2 has been hypothesized to induce an angiogenic response, whereas, in the absence of VEGF, Ang-2 leads to vessel regression [17–20]. Use of VEGF or (and) angiopoietin inhibitors to suppress angiogenesis has shown that the order of anti-angiogenesis is angiopoietin inhibitor < VEGF inhibitor < angiopoietin inhibitor + VEGF inhibitor [21]. Studies have shown that VEGF level is up-regulated in the peritoneal membrane of long-standing PD patients, resulting in angiogenesis of the peritoneum [3]. Furthermore, VEGF has been found in PD effluent, and its level positively correlates with vessel density [22, 23]. Functional inhibitors of VEGF and vascular endothelial growth factor receptors (VEGFRs) have been approved for the inhibition of angiogenesis. However, as yet, they cannot completely prevent angiogenesis. Thus, it is important to understand further the molecular mechanisms underlying not only VEGF–VEGFR signaling but also other regulation of angiogenesis [24]. In our study we found increasing Ang-2 and VEGF levels, as well as decreasing Tie-2 level, but normal Ang-1 level in the peritoneum, accompanied by angiogenesis under conditions of uremia in the dialysis group. The up-regulation of Ang-2 or VEGF was positively related to peritoneal angiogenesis, while Tie-2 was negatively related. Tie-2, as a receptor of angiopoietin, might function as a negative feedback to Ang-2 in regulating peritoneal angiogenesis. No Ang-1 level changed in any of the groups, as it mainly serves as a vascular stabilization factor. No significant difference in Ang-2 level was found among the PD groups, but the tendency of the level increase was consistent with angiogenesis. We wonder whether a larger sample size would show significant differences or some level of pro-angiogenic growth factor continuously promoting peritoneal angiogenesis. This would need further study. The tendency of low levels of VEGF found in the 56 days PD group was consistent with no difference in vessel density between the 56 days PD and 28 days PD groups. This again confirmed that VEGF played the central role in angiogenesis [21]. However, our study was done on an animal model of chronic PD for a maximum of 56 days and with a limited number of rats. The tendency of the VEGF level needs further study. Long-term and a larger sample size would be more persuasive to prove this hypothesis.
In conclusion, our study confirmed angiogenesis and fibrosis changes of the peritoneum as a result of uremic status and PD therapy in the uremic PD rat model. Increased levels of Ang-2, as well as a reduction in the level of Tie-2, under conditions of uremia and PD therapy correlated with peritoneal angiogenesis and functional deterioration. With the increased understanding of the angiopoietin/Tie-2 system involved in peritoneal angiogenesis, therapeutic maneuvers to target the angiopoietin/Tie-2 pathways may offer exciting new approaches to the preservation of the peritoneum.
References
Krediet RT, Lindholm B, Rippe B (2000) Pathophysiology of peritoneal membrane failure. Perit Dial Int 20 [Suppl 4]:S22–S42
De Vriese AS, Mortier S, Lameire NH (2001) Neoangiogenesis in the peritoneal membrane: does it play a role in ultrafiltration failure? Nephrol Dial Transplant 16:2143–2145
Saxena R (2008) Pathogenesis and treatment of peritoneal membrane failure. Pediatr Nephrol 23:695–703
Ramsauer M, D’Amore PA (2002) Getting Tie(2)d up in angiogenesis. J Clin Invest 110:1615–1617
Koh GY, Kim I, Kwak HJ, Yun MJ, Leem JC (2002) Biomedical significance of endothelial cell specific growth factor, angiopoietin. Exp Mol Med 34:1–11
Pereira BJ, Poutsiaka DD, King AJ, Strom JA, Narayan G, Levey AS, Dinarello CA (1992) In vitro production of interleukin-1 receptor antagonist in chronic renal failure, CAPD and HD. Kidney Int 42:1419–1424
Mateijsen MA, van der Wal AC, Hendriks PM, Zweers MM, Mulder J, Struijk DG, Krediet RT (1999) Vascular and interstitial changes in the peritoneum of CAPD patients with peritoneal sclerosis. Perit Dial Int 19:517–525
Chiang SC, Cheng CH, Moulton KS, Kasznica JM, Moulton SL (2000) TNP-470 inhibits intraabdominal adhesion formation. J Pediatr Surg 35:189–196
Tugues S, Fernandez-Varo G, Munoz-Luque J, Ros J, Arroyo V, Rodes J, Friedman SL, Carmeliet P, Jimenez W, Morales-Ruiz M (2007) Antiangiogenic treatment with sunitinib ameliorates inflammatory infiltrate, fibrosis, and portal pressure in cirrhotic rats. Hepatology 46:1919–1926
Margetts PJ, Gyorffy S, Kolb M, Yu L, Hoff CM, Holmes CJ, Gauldie J (2002) Antiangiogenic and antifibrotic gene therapy in a chronic infusion model of peritoneal dialysis in rats. J Am Soc Nephrol 13:721–728
Kalluri R, Sukhatme VP (2000) Fibrosis and angiogenesis. Curr Opin Nephrol Hypertens 9:413–418
Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, Newman GR, Mackenzie RK, Williams GT (2002) Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol 13:470–479
Witzenbichler B, Maisonpierre PC, Jones P, Yancopoulos GD, Isner JM (1998) Chemotactic properties of angiopoietin-1 and -2, ligands for the endothelial-specific receptor tyrosine kinase Tie2. J Biol Chem 273:18514–18521
Valenzuela DM, Griffiths JA, Rojas J, Aldrich TH, Jones PF, Zhou H, McClain J, Copeland NG, Gilbert DJ, Jenkins NA, Huang T, Papadopoulos N, Maisonpierre PC, Davis S, Yancopoulos GD (1999) Angiopoietins 3 and 4: diverging gene counterparts in mice and humans. Proc Natl Acad Sci U S A 96:1904–1909
Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD (1996) Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 87:1161–1169
Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD (1996) Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87:1171–1180
Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD (1997) Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277:55–60
Guo P, Imanishi Y, Cackowski FC, Jarzynka MJ, Tao HQ, Nishikawa R, Hirose T, Hu B, Cheng SY (2005) Up-regulation of angiopoietin-2, matrix metalloprotease-2, membrane type 1 metalloprotease, and laminin 5 gamma 2 correlates with the invasiveness of human glioma. Am J Pathol 166:877–890
Korff T, Kimmina S, Martiny-Baron G, Augustin HG (2001) Blood vessel maturation in a 3-dimensional spheroidal coculture model: direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates VEGF responsiveness. FASEB J 15:447–457
Roviezzo F, Tsigkos S, Kotanidou A, Bucci M, Brancaleone V, Cirino G, Papapetropoulos A (2005) Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. J Pharmacol Exp Ther 314:738–744
Takagi H, Koyama S, Seike H, Oh H, Otani A, Matsumura M, Honda Y (2003) Potential role of the angiopoietin/tie2 system in ischemia-induced retinal neovascularization. Invest Ophthalmol Vis Sci 44:393–402
Selgas R, del Peso G, Bajo MA, Molina S, Cirugeda A, Sanchez-Tomero JA, Castro MJ, Castro MA, Vara F (2001) Vascular endothelial growth factor (VEGF) levels in peritoneal dialysis effluent. J Nephrol 14:270–274
Combet S, Miyata T, Moulin P, Pouthier D, Goffin E, Devuyst O (2000) Vascular proliferation and enhanced expression of endothelial nitric oxide synthase in human peritoneum exposed to long-term peritoneal dialysis. J Am Soc Nephrol 11:717–728
Shibuya M (2008) Vascular endothelial growth factor-dependent and -independent regulation of angiogenesis. BMB Rep 41:278–286
Acknowledgments
The research was funded by the National Natural Science Foundation of China (30600290) and the Science and Technology Commission of Shanghai Municipality (07QA14040).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yuan, J., Fang, W., Ni, Z. et al. Peritoneal morphologic changes in a peritoneal dialysis rat model correlate with angiopoietin/Tie-2. Pediatr Nephrol 24, 163–170 (2009). https://doi.org/10.1007/s00467-008-0944-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-008-0944-5