Abstract
We compared, in a randomized controlled trial, the efficacy of a regimen based on intravenous (i.v.) cyclophosphamide therapy with a combination of i.v. dexamethasone and oral cyclophosphamide therapy in inducing remission in patients with steroid-resistant nephrotic syndrome (SRNS). During April 2001 to December 2003, 52 consecutive patients with idiopathic SRNS, normal renal function and renal histology findings showing minimal change disease, focal segmental glomerulosclerosis or mesangioproliferative glomerulonephritis were enrolled into the study. Patients in group I received i.v. injection of cyclophosphamide once a month for 6 months and prednisolone on alternate days. Those in group II received i.v. treatment with dexamethasone (initially on alternate days, later fortnightly and monthly; total 14 doses), oral cyclophosphamide therapy (for 3 months) and prednisolone on alternate days. Data from 49 patients (26 in group I, 23 in group II) were analyzed; their clinical and biochemical features were similar at inclusion. Following treatment, complete remission was seen in 53.8% and 47.8% patients in groups I and II, respectively (P = 0.6). Long-term follow up showed favorable outcome in 14 (53.8%) patients in group I, and 9 (39.1%) in group II. Chief adverse effects, including cushingoid features and serious infections, were similar in both groups. Patients receiving i.v. dexamethasone therapy commonly showed hypertension and hypokalemia, while vomiting and reversible alopecia occurred in those receiving i.v. treatment with cyclophosphamide. In patients with SRNS, the efficacy of treatment intravenously with cyclophosphamide and orally with prednisolone was similar to the combination of dexamethasone intravenously, orally administered cyclophosphamide and prednisolone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The treatment of patients with steroid-resistant nephrotic syndrome (SRNS) is difficult [1]. A number of medications have been used for the treatment of these patients, with variable results. Long-term treatment with calcineurin inhibitors is effective in a significant proportion of patients, but its cost and risk of nephrotoxicity limits its use as the first line agent [2–4]. Tune et al. proposed the intravenous (i.v.) use of high doses of corticosteroids combined with oral administration of cyclophosphamide with satisfactory results [5, 6]. This regimen requires multiple hospital admissions and monitoring for corticosteroid side effects. The efficacy of i.v. treatment with cyclophosphamide in patients with lupus nephritis and other vasculitides has led to its use in patients with SRNS [7, 8].
We have previously shown that a modified regimen requiring less frequent i.v. administration of corticosteroids achieved outcomes in SRNS, similar to that reported by Tune et al. [5, 9]. While there are no controlled trials that have compared the efficacy of intravenously administered methylprednisolone with intravenously administered dexamethasone, we reported that these agents had similar efficacy in inducing remission [10]. Furthermore, a small proportion of patients showing lack of response to i.v. treatment with dexamethasone showed remission of proteinuria with six i.v. doses of once-monthly cyclophosphamide [11]. The latter regimen is apparently convenient and requires fewer hospital admissions than does the former.
Since there are no prospective studies comparing the efficacy of these regimens, we conducted this randomized controlled trial to compare the efficacy and safety of initial treatment with intravenously administered cyclophosphamide and orally administered prednisolone versus the combination of i.v. dexamethasone, oral cyclophosphamide and prednisolone treatment in inducing remission in patients with idiopathic SRNS.
Patients and methods
Consecutive patients of idiopathic SRNS, between 1 year old and 18 years-old, with renal histology findings suggestive of minimal change disease (MCD), focal segmental glomerulosclerosis (FSGS) or mesangioproliferative glomerulonephritis (MesPGN) presenting to this hospital between April 2001 and December 2003 were eligible. Subjects with secondary nephrotic syndrome, e.g. lupus nephritis, immunoglobulin (Ig)A nephropathy, vasculitis and hepatitis B antigenemia, patients with renal dysfunction [estimated glomerular filtration rate (GFR) below 60 ml/min per 1.73 m2 body surface area] [12], and those previously treated with calcineurin inhibitors or immunosuppressive agents other than oral corticosteroids were excluded. The treatment protocol was approved by the Departmental Review Board. Informed, written parental consent was obtained prior to the study.
Nephrotic syndrome was defined as presence of nephrotic range proteinuria (> 1 g/m2 per day or 3+ or more on dipstick test), hypoalbuminemia (< 2.5 g/dl), and edema. Initial steroid resistance was defined as failure of the condition to respond to oral treatment with prednisolone at a dose of 2 mg/kg per day for 4 weeks [13]. Patients whose condition responded initially but failed to respond to 4-weeks’ daily treatment with prednisolone in subsequent relapses were labeled as late resistance.
Randomization
Stratified randomization, in blocks of four, was done separately with computer-generated numbers to allocate patients with initial and late steroid resistance randomly into groups I and II. Allocation was concealed in sealed opaque envelopes, which were opened by an associate not involved in the study.
Treatment
Group I patients received i.v. pulses of cyclophosphamide at a dose of 500 mg/m2 (maximum single dose 1 g) once every month for 6 months in the induction phase. The dosage was increased by 125 mg/m2 per month to a maximum of 750 mg/m2 if there had been no reduction in proteinuria by 3 months. Mercaptoethane sulfonate sodium (MESNA) was co-administered intravenously if the dose of cyclophosphamide was 750 mg/m2 or if there were features of bladder irritability or hematuria during a previous infusion. All subjects received prophylaxis for emesis with ondansetron (0.45 mg/kg, intravenously). Total leukocyte counts were estimated 4–7 days before the next i.v. pulse; treatment was deferred if counts were fewer than 3,000/mm3 or in the presence of systemic infection. The patients also received oral treatment with prednisolone at a dose of 1.5 mg/kg on alternate days for the first month, 1.25 mg/kg the next month and 1 mg/kg for 4 months (Table 1). During the 12-month maintenance phase, alternate-day therapy with prednisolone was continued (Table 1).
Group II patients received dexamethasone intravenously at a dose of 5 mg/kg (maximum single dose 150 mg) initially on alternate days, then fortnightly and finally once a month in the induction phase (Table 1). They also received cyclophosphamide orally for 12 weeks during the induction phase. Prednisolone was administered, during both phases, as in group I.
In addition to specific therapy, all subjects also received treatment with enalapril at a dose of 0.3 mg/kg daily in two divided doses; frusemide was used for control of edema, if necessary. None of the patients received lipid-lowering agents. Relapses during the maintenance phase were treated orally with prednisolone at a dose of 2 mg/kg per day for 2 weeks, followed by 1.5 mg/kg on alternate days for another 4 weeks, with subsequent tapering. The parents were instructed to examine the morning urine protein by dipstick test every other day and record the results in a diary. Blood counts and levels of urea, creatinine, albumin and cholesterol were measured every month until 6 months and then 3–4 monthly. Urine was examined for spot protein-to-creatinine ratios (Up/Uc, milligrams per milligram) at each visit. The height and weight standard deviation scores (SDS) [14] were recorded at inclusion and end of therapy.
Side effects of medications, including hypertension, arrhythmia, dyselectrolytemia, infections, cushingoid features (moon facies, truncal obesity, cutaneous striae and facial plethora), subcapsular cataract, features of steroid encephalopathy, vomiting, alopecia, skin rash and hemorrhagic cystitis, were recorded.
Outcome
Short-term outcome was assessed at the end of the 6-months induction phase. Complete remission was defined as negative findings for urine protein or a trace by dipstick test for 3 consecutive days or Up/Uc < 0.2, serum albumin > 2.5 g/dl and no edema. Partial remission was 1 + /2 + proteinuria by dipstick test or Up/Uc between 0.2 and 2.0, serum albumin > 2.5 g/dl and no edema. Non-response was 3 + /4 + proteinuria by dipstick test or Up/Uc of more than 2.0, serum albumin < 2.5 g/dl or edema. Failure of therapy was defined as: (a) non-response at 6 months, or (b) failure of the patient to complete the treatment due to either serious side effects (steroid-induced encephalopathy, seizures or arrhythmias) or occurrence of more than one episode of serious systemic infection (pneumonia, sepsis, peritonitis, cellulitis or meningitis). These subjects were excluded from further analysis and offered alternative treatment.
Long-term outcome was determined at the end of the maintenance phase, or until the beginning of alternative treatment, whichever was earlier. The outcome was considered favorable if patients continued to show complete or partial remission or steroid-sensitive relapses. Persistent nephrotic range proteinuria or GFR below 60 ml/min per 1.73 m2 was an unfavorable outcome.
Statistical analysis
Data are expressed as medians (ranges); statistical analysis was done with Wilcoxon rank sum test for continuous variables and chi-square tests (for discrete variables). Stepwise logistic regression analysis was done to identify variables that could affect response; P < 0.05 was considered significant.
Results
Of 68 eligible patients with idiopathic SRNS, secondary to MCD, FSGS or MesPGN, 16 were excluded, since they had received previous therapy with cyclophosphamide (eight patients), cyclosporine (five patients) and levamisole (three patients). Of those included, 27 (19 boys) were randomly allocated to group I, and 25 (16 boys) to group II. Three patients (one in group I and two in group II) were excluded during the first 3 months due to non-compliance with treatment; data from 49 patients was finally analyzed (Fig. 1).
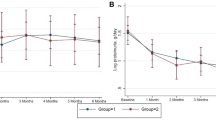
Course in group I (pulse treatment with cyclophosphamide, prednisolone) and group II (pulse treatment with dexamethasone, cyclophosphamide orally, prednisolone). Short-term outcome was assessed at the end of 6 months of intensive therapy, and long-term outcome was assessed 12 months later. The outcomes were similar in both treatment regimens. CR complete remission, PR partial remission, NR no response
Baseline features
Eighteen (36.7%) patients had initial resistance and 31 (63.3%) late resistance. Renal histology showed MCD in 24 (49%), FSGS in 14 (28.6%) and MesPGN in 11 (22.4%). Although patients’ age at onset of nephrotic syndrome and inclusion in the study were higher in group II, the difference was not statistically significant (P = 0.11). Other features, including duration of resistance prior to inclusion, proportion of patients with initial resistance, renal histology and biochemical features, were comparable (Table 2).
Short-term outcome
Following i.v. therapy with cyclophosphamide (group I), 14 (53.8%) patients had complete remission, two (7.7%) partial remission and ten (38.5%) either showed no response (seven patients) or had discontinued their therapy due to multiple episodes of serious infections (three patients). Similar outcomes were seen in patients treated with the combination of dexamethasone pulses and orally administered cyclophosphamide, where complete remission was seen in 11 (47.8%) and partial remission in two (8.7%) patients. Ten (43.5%) patients in group II either showed no response (five patients) or had discontinued their treatment due to steroid encephalopathy (one) or multiple episodes of serious infections (four patients). Kaplan–Meier analysis showed similar proportions of patients with complete remission in the two groups (Fig. 2).
Kaplan–Meier analysis showing no difference in the proportion of patients not achieving complete remission during induction; log rank test, P = 0.6. Group I (cyclophosphamide intravenously and prednisolone) represented by solid line, group II (dexamethasone intravenously, cyclophosphamide orally and prednisolone) by small dots
Following 6 months of induction therapy, the median blood levels of albumin increased, and cholesterol levels and Up/Uc ratio reduced within each group (Table 3). There were, however, no significant differences in these levels and estimated GFR between the groups.
Factors affecting response to treatment
Patients showing complete or partial remission had been younger at onset of nephrotic syndrome (median age 27.5 months and 48 months in groups I and II, respectively) than those with no response (respective median ages 43 months and 68 months); however, these differences were not significant. Similarly, in both groups, patients showing complete or partial remission were approximately 20 months younger at inclusion into the study than those not responding. In group I, partial or complete remission was achieved in six of ten (60%) patients with initial steroid resistance and ten of 16 (62.5%) patients with late steroid resistance. The respective remission rates among patients with initial and late resistance in group II were five of eight (62.5%) and eight of 15 (53.3%), respectively. Nine of 13 (69.2%) patients in group I, and seven of 11 (63.6%) in group II with MCD showed complete or partial remission. Among patients with FSGS and MesPGN, seven of 13 (53.8%) in group I and six of 12 (50%) in group II showed remission. The renal histology and presence of tubulointerstitial changes were not related to response to treatment.
Stepwise logistic regression analysis showed that female gender [odds ratio (OR) 7.5, 95% confidence interval (95% CI) 1.2–46.4; P = 0.03] was the only significant predictor of complete or partial remission. Factors not predictive of response included the patient’s age at onset (OR 0.99, 95% CI 0.97–1.0; P = 0.4), histology (OR 0.9, 95% CI 0.3–2.4; P = 0.8), baseline blood levels of albumin (OR 2.4, 95% CI 0.8–6.7; P = 0.1) and cholesterol (OR 1.0, 95% CI 0.99–1.01; P = 0.5), creatinine clearance (OR 0.99, 95% CI 0.96–1.03; P = 0.6) and urine protein-to-creatinine ratio (OR 1, 95% CI 0.8–1.3; P = 0.8). The response to therapy was similar in patients with initial or late resistance (OR 2.4, 95% CI 0.3–17.7; P = 0.4).
Long-term course
In both groups, patients with complete remission (14 in group I and 11 in group II) showed sustained remission or steroid-sensitive relapses. Of the 14 patients with complete remission in group I, 11 (78.6%) were still in remission, while three patients had had steroid-sensitive relapses, at the last follow-up examination. In the two patients with partial remission, one patient’s condition became steroid resistant. Similarly, in group II, of the 11 patients with complete remission, nine (81.8%) had sustained remission while two had steroid-sensitive relapses at the last follow-up examination. The condition of both patients with partial remission became steroid resistant. All patients that had not responded at the end of 6-months’ initial therapy in either group continued to show nephrotic-range proteinuria, and one patient each from groups I and II showed deteriorating kidney function (CKD stages III–IV) on follow up. Favorable outcome was seen in 14 (53.8%) patients in group I and 11 (47.8%) in group II at long-term follow-up examination (P = 0.2).
Adverse effects
Patients in both groups showed cushingoid features and were at risk for systemic infections (Table 4). Pneumonia (six patients) was the commonest infection, followed by peritonitis (three patients), cellulitis (three patients) and meningitis (two patients). Most infections (85.7%) occurred in the first 3 months of treatment. Intravenous administration of dexamethasone was associated with hypertension in ten patients and hypokalemia (serum potassium < 3.5 mEq/l) in seven (30.4%). Adverse effects specifically associated with i.v. use of cyclophosphamide included vomiting, reversible alopecia, hemorrhagic cystitis and leukopenia.
Discussion
The management of SRNS is challenging, with patients at risk for complications of unremitting nephrotic syndrome and end-stage renal disease. Multiple therapies have been proposed for treatment of these patients [3]. Tune et al. showed beneficial results in 65% patients treated with multiple i.v. pulses of methylprednisolone, cyclophosphamide orally for 8–12 weeks, and tapering doses of prednisone over 30 months [5, 6]. In view of significant steroid toxicity and need for several admissions for the infusions, many centers have used shorter protocols with comparable benefit, ranging between 30% and 70% [3, 15]. A study from this center, using an abbreviated protocol of steroids intravenously and cyclophosphamide orally, showed remission in 25 of 59 (42.4%) patients within the first 6 months; 64.7% of these were in remission at the 2 year follow-up examination [9]. Since treatment with methylprednisolone is expensive in India, its replacement with i.v. therapy with dexamethasone, a less expensive agent, has been shown to be associated with similar benefits [10].
Intravenous therapy with cyclophosphamide, administered monthly for 6 months, has been reported to be effective in inducing remission in patients with SRNS. A randomized trial of 13 patients with SRNS that compared intravenous use and oral use of cyclophosphamide showed beneficial results in 100% and 25% patients, respectively [16]. In another report, 65% of 20 patients with FSGS treated with i.v. pulse therapy showed complete remission [8]. We have previously reported our prospective experience on the efficacy of a similar regimen in 24 patients with SRNS. Intravenous administration of cyclophosphamide resulted in complete or partial remission in 58% patients at 6 months. It was notable that all subjects with partial remission had recurrence of nephrotic-range proteinuria, and, on long-term follow-up, only 21% of the entire group showed sustained remission [11].
While multiple case series show the efficacy of regimens using steroids intravenously and cyclophosphamide orally [5, 6, 9, 10, 15], and cyclophosphamide intravenously [8, 11, 16], there are no controlled trials on their comparative efficacy. In the randomized controlled study reported here, both regimes were comparable in inducing remission of nephrotic syndrome. At the end of induction treatment, 61.5% patients receiving cyclophosphamide intravenously and 56.5% given dexamethasone intravenously and cyclophosphamide orally, were in complete or partial remission. At follow-up examination 12-months later, 53.8% patients in the former group and 39.1% in the latter were either in remission or had had steroid-sensitive relapses.
While the immediate outcome was similar, long-term results on intravenously administered cyclophosphamide in our study are better than those reported previously from this center [11]. These differences are most likely due to inclusion of patients with different characteristics in the two studies. The study reported here included fresh patients with SRNS who had previously not been treated with immunosuppressive medications, apart from prednisolone, while the previous report included subjects who had failed to respond to six i.v. pulses of dexamethasone [11]. Furthermore, the proportion of patients whose condition was initially steroid resistant was 36.7% in the study reported here compared with 75% in the previous report [11]. Since patients with an initially steroid-resistant condition are considered less likely to respond to treatment [7, 8, 11, 17], the inclusion of more subjects with late resistance in our study here might have influenced the results.
Results from randomized controlled studies suggest that oral therapy with cyclophosphamide is not effective in patients with SRNS [18]. However, a recent report from South Africa, on Indian children with steroid-resistant FSGS, showed complete remission in 69% patients [19]. The corresponding rates of remission in black subjects were 20.2%, similar to that reported previously [18]. The reasons for better response to cyclophosphamide in patients of Indian ancestry are not clear but might be ascribed to unknown genetic or pharmacokinetic factors. On the other hand, compared with Caucasians, patients of African-American descent show higher incidence of FSGS and unsatisfactory response to treatment and outcome [20, 21].
In the trial documented here, most responders (78.6%) in group I and group II (81.8%) attained remission within the first 4 months of therapy. This was expected, as the subjects in both groups had received intense immunosuppression during the first 6 months. Previous studies suggest that remission of proteinuria following i.v. treatment with cyclophosphamide usually occurs with 3–5 doses of the drug [7, 8, 11, 15]. Similarly, studies with i.v. pulses of steroids have shown that most patients who respond do so by the second month of therapy [5, 9]. In the our study, occurrence of complete remission was associated with a favorable long-term outcome. Most patients with partial remission showed recurrence of steroid resistance, confirming that the occurrence of partial remission with either of these regimens is not a satisfactory outcome [5, 11, 15].
There was no difference between the responders and non-responders in terms of their baseline clinical, laboratory or histological characteristics. While patients with MCD and late resistance are believed to respond satisfactorily to immunosuppressive therapy [7, 22], we could not show a differential response based on histology or pattern of resistance, perhaps due to the small number of subjects. While there was a trend towards an earlier age of onset in patients showing complete or partial remission than in those with no response, differences between the groups were not significant. Similar findings, showing better outcome in patients that were younger at onset, were reported by Mongeau et al. [23]. An interesting, but unexplained, finding in our study was that girls showed significantly better response to therapy than boys (OR 7.5, P = 0.03). While previous reports have suggested an unsatisfactory outcome in boys [22], others have not shown significant differences based on gender [24, 25].
The frequency of serious side effects associated with both these therapies is a cause for concern. Patients in both groups showed evidence of steroid toxicity and were at significant and comparable risk of systemic infections. Despite routine use of a single dose of ondansetron, most subjects had vomiting following infusion of cyclophosphamide. The risk of hemorrhagic cystitis, leukopenia and alopecia was comparable to that reported previously [26]. As reported previously, transient and rarely sustained hypertension and hypokalemia were common side effects of i.v. treatment with steroids [9, 10]. Long-term adverse effects of therapy with high doses of cyclophosphamide, including the risks of malignancy and gonadotoxicity, were not evaluated. However, in none of the patients was the cumulative dose of 250 mg/kg, which is believed to be gonadotoxic, exceeded [26].
Results of this prospective study show that therapy with cyclophosphamide intravenously and prednisolone orally is as effective as the combination of i.v. dexamethasone, oral cyclophosphamide and prednisolone therapy in its ability to induce remission in patients with SRNS. Although formal cost comparisons were not done, the administration of six monthly pulses of cyclophosphamide is, perhaps, convenient and less expensive than the need for more than twice the number of hospitalizations required for i.v. dexamethasone administration. It is emphasized that the rates of favorable long-term outcomes with either of these agents were clearly lower than those reported with the use of cyclosporin [27] and tacrolimus [28]. Even in our study, five of seven patients that did not respond in groups I and II showed complete remission following treatment with cyclosporin. However, long-term therapy with calcineurin inhibitors is expensive and might be associated with significant side effects, including cosmetic effects, nephrotoxicity, neurotoxicity and medication dependency [2, 27].
There is a need to examine treatments for SRNS that are safe and effective and, in the context of developing regions, also cost-effective. Our findings suggest that i.v. treatment with cyclophosphamide is convenient and promising for such patients. It would be desirable to compare, in a multicentric, randomized, controlled trial, the results of initial treatment using either intravenously administered cyclophosphamide or a calcineurin inhibitor (e.g. tacrolimus). Patients in both groups should receive concomitant therapy with steroids on alternate days, an angiotensin-converting enzyme inhibitor and a calcium supplement. Such a study would require stratification for patients with initial and late steroid resistance and be adequately powered to compare the long-term efficacy and safety of the treatments.
References
Eddy AA, Symons JM (2003) Nephrotic syndrome in childhood. Lancet 362:629–639
Niaudet P, Fuchshuber A, Gagnadoux MF, Habib R, Broyer M (1997) Cyclosporine in the therapy of steroid-resistant idiopathic nephrotic syndrome. Kidney Int Suppl 58:S85–S90
Habashy D, Hodson EM, Craig JC (2003) Interventions for steroid-resistant nephrotic syndrome: a systematic review. Pediatr Nephrol 18:906–912
Bhimma R, Adhikari M, Asharam K, Connolly C (2006) Management of steroid-resistant focal segmental glomerulosclerosis in children using tacrolimus. Am J Nephrol 26:544–551
Tune BM, Kirpekar R, Sibley RK, Reznik VM, Griswold WR, Mendoza SA (1995) Intravenous methylprednisolone and oral alkylating agent therapy of prednisone-resistant pediatric focal segmental glomerulosclerosis: a long-term follow-up. Clin Nephrol 43:84–88
Tune BM, Lieberman E, Mendoza SA (1996) Steroid resistant focal segmental glomerulosclerosis; a treatable disease. Pediatr Nephrol 10:772–778
Rennert WP, Kala UK, Jacobs D, Goetsch S, Verhaart S (1999) Pulse cyclophosphamide for steroid-resistant focal segmental glomerulosclerosis. Pediatr Nephrol 13:113–116
Gulati S, Kher V (2000) Intravenous pulse cyclophosphamide—a new regime for steroid resistant focal segmental glomerulosclerosis. Indian Pediatr 37:141–148
Hari P, Bagga A, Jindal N, Srivastava RN (2001) Treatment of focal glomerulosclerosis with pulse steroids and oral cyclophosphamide. Pediatr Nephrol 16:901–905
Hari P, Bagga A, Mantan M (2004) Short term efficacy of intravenous dexamethasone and methylprednisolone therapy in steroid resistant nephrotic syndrome. Indian Pediatr 41:993–1000
Bajpai A, Bagga A, Hari P, Dinda A, Srivastava RN (2003) Intravenous cyclophosphamide in steroid-resistant nephrotic syndrome. Pediatr Nephrol 18:351–356
Schwartz GJ, Haycock GB, Edelman CM, Spitzer A (1976) A simple measure of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58:259–263
Indian Pediatric Nephrology Group, Indian Academy of Pediatrics (2001) Consensus statement on management of steroid sensitive nephrotic syndrome. Indian Pediatr 38:975–986
National Center for Health Statistics (1978) Centers for Disease Control NCHS growth curves for children: birth–18 years. Washington DC: US Government Printing Office (Series 11, 165. DHEW publication (PHS) 78 1650)
Adhikari M, Bhimma R, Coovadia HM (1997) Intensive pulse therapies for focal glomerulosclerosis in South African children. Pediatr Nephrol 11:423–8
Elhence R, Gulati S, Kher V, Gupta A, Sharma RK (1994) Intravenous pulse cyclophosphamide—a new regime for steroid resistant minimal change nephrotic syndrome. Pediatr Nephrol 8:1–3
Tarshish P, Tobin JN, Bernstein J, Edelmann CM Jr (1997) Prognostic significance of the early course of minimal change nephrotic syndrome: report of the International Study of Kidney Disease in Children. J Am Soc Nephrol 8:769–776
Tarshish P, Tobin JN, Bernstein J, Edelman CM Jr (1996) Cyclophosphamide does not benefit patients with focal segmental glomerulosclerosis: a report of the International Study of Kidney Diseases in Children. Pediatr Nephrol 10:590–593
Bhimma R, Adhikari M, Asharam K (2006) Steroid-resistant nephrotic syndrome: the influence of race on cyclophosphamide sensitivity. Pediatr Nephrol 21:1847–1853
Ingulli E, Tejani A (1991) Racial differences in the incidence and renal outcome of idiopathic focal segmental glomerulosclerosis in children. Pediatr Nephrol 5:393–397
Boyer O, Moulder JK, Somers MJG (2007) Focal and segmental glomerulosclerosis in children: a longitudinal assessment. Pediatr Nephrol 22:1159–1166
Gulati S, Kher V, Sharma RK, Gupta A (1994) Steroid response pattern in Indian children with nephrotic syndrome. Acta Paediatr 83:530–533
Mongeau JG, Corneille L, Robitaille P, O’ Regan S, Pelletier P (1981) Primary nephrosis in childhood associated with focal segmental glomerulosclerosis: is long term prognosis that severe? Kidney Int 20:743–746
Hiraoka M, Tsukahara H, Haruki S, Hayashi S, Takeda N, Miyagawa K, Okuhara K, Suehiro F, Ohshima Y, Mayumi M (2000) Older boys benefit from higher initial prednisolone therapy for nephrotic syndrome The West Japan Cooperative Study of Kidney Disease in Children. Kidney Int 58:1247–1252
Constantinescu AR, Shah HB, Foote EF, Weiss LS (2000) Predicting first year relapses in children with nephrotic syndrome. Pediatrics 105:492–495
Latta K, Von Schnakenburg C, Ehrich JH (2001) A meta-analysis of cytotoxic treatment for frequently relapsing nephrotic syndrome in children. Pediatr Nephrol 16:271–282
Cattran DC, Alexopoulos E, Heering P, Hoyer PF, Johnston A, Meyrier A, Ponticelli C, Saito T, Choukroun G, Nachman P, Praga M, Yoshikawa N (2007) Cyclosporine in idiopathic glomerular disease associated with the nephritic syndrome: workshop recommendations. Kidney Int 72:1429–1447
Gulati S, Prasad N, Sharma RK, Kumar A, Gupta A, Baburaj VP (2008) Tacrolimus: a new therapy for steroid resistant nephrotic syndrome in children. Nephrol Dial Transplant 23:910–913
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mantan, M., Sriram, C.S., Hari, P. et al. Efficacy of intravenous pulse cyclophosphamide treatment versus combination of intravenous dexamethasone and oral cyclophosphamide treatment in steroid-resistant nephrotic syndrome. Pediatr Nephrol 23, 1495–1502 (2008). https://doi.org/10.1007/s00467-008-0860-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-008-0860-8