Abstract
Mutations in PKHD1 cause autosomal recessive polycystic kidney disease (ARPKD). We produced a mouse model of ARPKD by replacing exons 1–3 of Pkhd1 with a lacZ reporter gene utilizing homologous recombination. This approach yielded heterozygous Pkhd1lacZ/+ mice, that expressed β-galactosidase in tissues where Pkhd1 is normally expressed, and homozygous Pkhd1lacZ/lacZ knockout mice. Heterozygous Pkhd1lacZ/+ mice expressed β-galactosidase in the kidney, liver, and pancreas. Homozygous Pkhd1lacZ/lacZ mice lacked Pkhd1 expression and developed progressive renal cystic disease involving the proximal tubules, collecting ducts, and glomeruli. In the liver, inactivation of Pkhd1 resulted in dilatation of the bile ducts and periportal fibrosis. Dilatation of pancreatic exocrine ducts was uniformly seen in Pkhd1lacZ/lacZ mice, with pancreatic cysts arising less frequently. The expression of β-galactosidase, Pkd1, and Pkd2 was reduced in the kidneys of Pkhd1lacZ/lacZ mice compared with wild-type littermates, but no changes in blood urea nitrogen (BUN) or liver function tests were observed. Collectively, these results indicate that deletion of exons 1–3 leads to loss of Pkhd1 expression and results in kidney cysts, pancreatic cysts, and biliary ductal plate malformations. The Pkhd1lacZ/lacZ mouse represents a new orthologous animal model for studying the pathogenesis of kidney cysts and biliary dysgenesis that characterize human ARPKD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autosomal recessive polycystic kidney disease (ARPKD) is a monogenic disorder that is characterized by cystic kidney disease and biliary dysgenesis [1]. The kidney cysts originate from the renal collecting ducts and produce nephromegaly and chronic renal failure. Neonates with the severe perinatal form of ARPKD have a high mortality rate and present with intrauterine kidney failure, oligohydramnios, Potter’s facies, and pulmonary hypoplasia. ARPKD can also present later in life with less severe cystic disease and a better prognosis. All ARPKD patients have liver involvement due to biliary dysgenesis, a ductal plate malformation characterized by aberrant development of the intrahepatic bile ducts. The liver abnormalities range from dilated, irregular bile ducts within the portal triad to more extensive disease, including hepatosplenomegaly and choledochal cysts (Caroli’s disease) [2]. Over time, the liver disease progresses to periportal fibrosis and portal hypertension.
ARPKD is caused by mutations in PKHD1 (polycystic kidney and hepatic disease 1) [3, 4]. Mutations have been found throughout the gene, and genotype–phenotype correlations have revealed that the presence of two truncating mutations causes perinatal lethal disease, whereas missense mutations are associated with longer-term survival [5]. The human PKHD1 gene is located on chromosome 6 and consists of 67 exons, with the longest open reading frame extending over 12 kb. The orthologous mouse Pkhd1 transcript is ∼14 kb and contains a start codon in exon 2. Although exons 1 and 2 have no homology to the human gene, exon 3 contains the site of one of the most common human mutations, T36M, and shows 91% homology to the human gene [4].
The function of the protein product of Pkhd1, called fibrocystin or polyductin, remains unclear. Its predicted structure suggests that it is a membrane protein similar to the hepatocyte growth factor receptor. Fibrocystin is located in the primary cilium, basal body, and apical plasma membrane [6]. Recent studies have shown that fibrocystin undergoes proteolytic cleavage, releasing a C-terminal fragment that translocates to the nucleus [7, 8]. Fibrocystin also participates in a ciliary complex with polycystin-2, which is mutated in autosomal dominant polycystic kidney disease (ADPKD) and is involved in flow-stimulated intracellular calcium signaling [9].
Generation of a Pkhd1 knockout mouse that mimics the human disease has been challenging. Deletion of exon 40 of Pkhd1 produces biliary dysgenesis but does not cause kidney abnormalities [10]. Deletion of exon 2 results in proximal tubule dilatation in older females, but males are unaffected and no involvement of the collecting ducts is observed [11]. Deletion of exons 3–4 of Pkhd1 produces cysts in the thick ascending limb and collecting duct cysts that usually appear after 3 months of age [12]. We targeted exons 1–3 of Pkhd1, which includes the site of the T36M mutation, to generate a model that may more closely replicate the human disease. Similar to humans with ARPKD, the mouse develops kidney cysts and ductal plate malformations and should be useful for our understanding of the pathogenesis of the disease. In addition, our model includes a lacZ reporter gene inserted at the Pkhd1 locus, which will assist in the understanding of Pkhd1 gene expression and promoter activity.
Materials and methods
Generation of Pkhd1lacZ mice
High-fidelity polymerase chain reaction (PCR) was used to amplify the proximal 2-kb Pkhd1 promoter from a bacterial artificial chromosome (BAC) containing the Pkhd1 gene (BAC354J18 from the Roswell Parker Cancer Institute (RPCI)-22 BAC genomic library). The PCR fragment was gel-purified and cloned into the KpnI and XbaI sites upstream of the lacZ reporter gene in the plasmid pnLacF [13]. A targeting vector was created using the plasmid osdupdel, which contains a floxed neomycin resistance gene and thymidine kinase cassette [14]. The Pkhd1/lacZ cassette was released from pnLacF and cloned into the KpnI and BamHI sites of osdupdel, creating the short (5’) arm of homology. Exons 4–5 of Pkhd1 were amplified from the BAC and cloned into the SpeI and SfiI sites of osdupdel, creating the long (3’) arm of homology. The plasmid was linearized with Spe1 and purified. Embryonic stem (ES) cells (129Sv) were transfected with the Pkhd1/lacZ knock-in construct by the University of Texas (UT) Southwestern Transgenic Core Facility. ES cell clones were selected with G418/ganciclovir. Positive clones were identified by PCR and confirmed by Southern blot analysis. Successfully targeted cells were injected into C57BL/6J blastocysts and transferred into Institute Cancer Research (ICR) pseudopregnant foster mothers. The resulting chimeric animals were backcrossed with C57BL/6J for six generations. Pkhd1lacZ/+ heterozygotes were intercrossed to generate homozygous Pkhd1lacZ/lacZ knockout mice. All experiments involving animals were conducted under the auspices of the UT Southwestern Institutional Animal Care and Use Committee.
Reverse transcriptase–polymerase chain reaction analysis
Total kidney RNA was extracted with Trizol reagent (Invitrogen). One microgram of RNA was treated with amp grade DNAaseI, and cDNA was synthesized with the use of Stratascript RT (Stratagene). PCR was performed with the following primers (conditions available on request):
Pkhd1 (exons 1–3): 5’-CATTTGAGGCACAAGGCTGACACA-3’, 5’-TATGGCCCTGCATCTGCTTCTGAT-3’; Pkhd1 (exons 10–11): 5’-GGGAGGTCGATGGTGCATAAG-3’, 5’-GATGTCCGTTCTTCCCCCAAG-3’;
Pkhd1 (exons 34–36): 5’-GACAGAGTCAGTCTTTCCATCGTT-3’, 5’-TTCGCATCAAGCAGTAGCAGG-3’; Pkhd1 (exons 53–54): 5’-AAGTCAAGGGCCATCACATC-3’, 5’ATGTTTCTGGTCAACAGCCC-3’; Pkd1: 5’-GGTAGAGGCTGGCTCAGA-3’, 5’-TGGGGTAGTGTAAATATGCG-3’; Pkd2: 5’-GTCGAAAACAGAGAAAACCAAC-3’, 5’-ATGATCTCACAGGCTGCCAGG-3’
X-Gal and histological staining
We measured expression of β-galactosidase by staining with 5-bromo-4-chloro-3-indoyl β D-galactosidase (X-Gal, Stratagene), as described previously [15]. Briefly, adult tissues were perfused with ice-cold phosphate-buffered saline (PBS) followed by PBS containing 2% paraformaldehyde. The kidneys were bisected and immersed in 2% paraformaldehyde for 1 h. After fixation, tissue samples were rinsed with PBS and incubated overnight at 4°C in PBS containing 30% sucrose. Cryoprotected samples were embedded in optimal cloning temperature (OCT) medium, frozen in liquid nitrogen, sectioned at 5–10 μm, and mounted on Vectabond-coated slides. The sections were incubated in staining solution [PBS containing 20 mM Tris-Cl (pH 7.4), 1.8 mM spermidine, 2 mM MgCl2, 0.02% Nonidet P-40, 0.01% sodium deoxycholate, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, and 1 mg/ml X-Gal]. Staining with X-Gal was performed at 37°C in the dark with continuous agitation. The sections were washed with PBS and counterstained with eosin (Sigma-Aldrich). The stained sections were rinsed with PBS and photographed under bright-field illumination using a Zeiss Axioplan 2 microscope. The paraffin-embedded sections were stained with hematoxylin and eosin according to standard protocols (UT Southwestern Molecular Pathology Core).
Antibody and lectin staining
Paraformaldehyde-fixed cryosections were stained with antibodies against aquaporin-3 (AQP3, Chemicon) and Na-K-Cl cotransporter 2 (NKCC2, Sigma) as described previously [16]. We conjugated the NKCC2 antibody, using a Zenon 488 labeling kit. Lectin staining was performed with fluorescein isothiocyanate-coupled Lotus tetragonolobus agglutinin and Dolichos biflorus agglutinin (LTA and DBA, Vector Laboratories, Burlingame, CA, USA).
Biochemical studies
Whole blood was collected from anesthetized mice via retro-orbital venous puncture. The samples were incubated on ice then centrifuged at 4,000 r.p.m. for 10 min. The serum was aspirated and stored at 4°C. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyltransferase (γGT) and blood urea nitrogen (BUN) were measured by the Mouse Metabolic Phenotyping Core at UT Southwestern Medical Center using a Vitros 250 chemical analyzer (Orthoclinical Diagnostics).
Results
Generation of Pkhd1 knockout mice
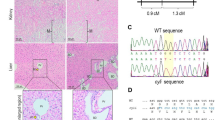
A targeting vector was designed to replace exons 1–3 of Pkhd1 with a lacZ reporter gene. The targeting vector contained 2,043 bp of the proximal Pkhd1 promoter linked to a nuclear-localized lacZ reporter gene, followed by exons 4–5 of Pkhd1 (Fig. 1a). The lacZ reporter gene contained a stop codon so that no fusion protein would be generated. ES cells were transfected with the plasmid and subjected to positive–negative selection with G418 and ganciclovir. Homologous recombination was confirmed by Southern blot analysis (Fig. 1b) and PCR using primers flanking the end of the targeting vector (Fig. 1c). Three successfully targeted ES cell clones were identified and injected into C57BL/6J blastocysts. Chimeric progeny were mated with C57BL/6J mice and backcrossed for six generations. Heterozygous Pkhd1lacZ/+ mice and homozygous Pkhd1lacZ/lacZ mice were fertile and were used for breeding. Matings between Pkhd1lacZ/+ heterozygotes produced the expected Mendelian ratios of homozygous mice. Figure 1d shows reverse transcriptase–polymerase chain reaction (RT-PCR) analysis of RNA extracted from the kidneys of wild-type, heterozygous, and homozygous mice. Primers from multiple regions of the gene were used to account for different Pkhd1 transcripts generated by alternative splicing. The homozygous Pkhd1lacZ/lacZ mice did not express transcripts containing any of the amplified regions, whereas the heterozygous and wild-type mice expressed transcripts containing all the regions. These results indicated that Pkhd1lacZ was a null allele. Semi-quantitative RT-PCR analysis was performed to determine if the expression of other genes involved in polycystic kidney disease was altered in Pkhd1lacZ/lacZ mice. Figure 1e shows that the expression of Pkd1 and Pkd2 was diminished in homozygous mice compared with heterozygous mice.
Pkhd1 knock-in strategy. aUpper Wild-type Pkhd1 locus showing proximal promoter (thick line) and exons 1–5 (boxes). MiddlePkhd1 targeting vector containing 2,043 bp of the proximal Pkhd1 promoter, lacZ reporter gene with nuclear localization signal, inverted floxed neomycin resistance gene, and Pkhd1 exons 4–5. Lower Recombinant locus showing replacement of Pkhd1 exons 1–3 with the lacZ reporter gene. The bar indicates the probe for Southern blot analysis, and arrows indicate PCR primers used to confirm targeting. BBclI. b Southern blot analysis of ES cell clones 5D6, 5B4, and 1E1. The 5.5 kb band is present in the ES cells and wild-type DNA. The 8.6 kb band is seen only in the correctly targeted ES cells. c PCR confirming the targeting of Pkhd1. The 2.3 kb product was generated with an internal and external primer. d RT-PCR analysis of Pkhd1 in the kidney. Primers were designed to amplify exons 1–3, 10–11, 53–54, and 34–36. Pkhd1 transcripts were detected in wild-type (+/+) and heterozygous (+/−) mice but not in homozygous mice (−/−). Gapdh was used as a positive control. e RT-PCR analysis of Pkd1 and Pkd2 in the kidneys at P60. Pkd1 and Pkd2 transcript levels were diminished in homozygous mutant mice compared with heterozygous mice. 28S RNA was used as a positive control
Pkhd1lacZ/lacZ mice develop cysts in proximal tubules, collecting ducts, and glomeruli
Heterozygous Pkhd1lacZ/+ mice had normal-appearing kidneys (not shown). Homozygous Pkhd1lacZ/lacZ mice also had normal kidney morphology at birth but subsequently developed kidney cysts. Cyst formation was first observed when the mice were 45 days of age and progressed during adulthood. Both tubular and glomerular cysts were seen in adult homozygous mice (Fig. 2b,c) as opposed to the normal kidney histology seen in age-matched wild-type controls (Fig. 2a). Dilated tubules were found in both the renal cortex and medulla, but macrocysts were primarily located near the corticomedullary junction. Tubules were considered cystic if their diameters exceeded three-times that of the normal diameter [17]. A total of 69 Pkhd1lacZ/lacZ mice were analyzed. Severe cystic kidney disease was observed in 14/14 (100%) of homozygous Pkhd1lacZ/lacZ mice by the time they were 9 months of age. Trichrome staining showed that the kidney cysts in Pkhd1lacZ/lacZ mice were surrounded by areas of increased interstitial fibrosis when the mice were compared with age-matched Pkhd1lacZ/+ mice (Fig. 2d–f). To identify the origins of the renal cysts, we stained the kidney sections with markers of specific nephron segments. The markers that were used were Lotus tetragonolobus agglutinin (LTA) for proximal tubules, Na-K-Cl cotransporter 2 (NKCC2) for thick ascending limbs, and aquaporin-3 (AQP3) for collecting ducts. Figure 2g shows multiple cortical cysts that were LTA-positive. Figure 2h shows that NKCC2 was absent in the cysts but was expressed in surrounding non-cystic tubules. Figure 2i shows a large cyst containing AQP3 in the basolateral membrane. These results indicated that the tubular cysts originated from proximal tubules and collecting ducts. Similar findings were observed in Pkhd1lacZ/lacZ mice after inbreeding on a 129SvEv background (data not shown). No sex differences were observed in the renal cystic phenotype of Pkhd1lacZ/lacZ mice.
Formation of kidney cysts in Pkhd1lacZ/lacZ mice. a Hematoxylin and eosin (H&E)-stained section of kidney from a 9-month-old wild-type mouse. b H&E-stained section of kidney from a 9-month-old Pkhd1lacZ/lacZ mouse. c Higher magnification image showing glomerular cysts and dilated tubules. d Trichrome-stained section of kidney from a 9-month-old Pkhd1lacZ/+ mouse. e Trichrome-stained section of kidney from a 9-month-old Pkhd1lacZ/lacZ mouse. f Higher magnification image showing interstitial fibrosis and kidney cysts. g–i Kidney sections from a Pkhd1lacZ/lacZ mouse stained (green) with LTA (g) or antibodies to NKCC2 (h) and AQP3 (i). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Bars represent 50 μm. cy cyst
Decreased Pkhd1 promoter activity in kidney cysts
We replaced exons 1–3 of Pkhd1 with a lacZ reporter gene containing a nuclear localization signal. The resulting Pkhd1lacZ knock-in mice expressed β-galactosidase in the nuclei of cells under the control of the endogenous Pkhd1 promoter and regulatory elements. Figure 3a shows the expression of β-galactosidase in the kidneys from an adult heterozygous Pkhd1lacZ/+ mouse. β-Galactosidase was highly expressed in the nuclei of medullary collecting ducts (Fig. 3b). In the cortex, β-galactosidase was expressed in glomerular parietal epithelial cells and proximal tubules (Fig. 3c). No expression of β-galactosidase was observed in non-epithelial cells in the kidney.
Pkhd1 promoter activity in kidney cysts. a–c X-Gal and eosin-stained kidney sections from Pkhd1lacZ/+ mice showing β-galactosidase expression in medullary tubules (b) and Bowman’s capsule (c). d X-Gal and eosin-stained sections from 45-day-old Pkhd1lacZ/lacZ mice, showing medullary and cortical cysts. e Higher magnification image of a glomerular cyst, showing absence of β-galactosidase expression. f Mildly dilated cortical collecting duct containing β-galactosidase-positive cells. g High-magnification image of a medullary cyst from a Pkhd1lacZ/lacZ mouse, showing β-galactosidase expression in cyst epithelial cells. h–j X-Gal and eosin-stained kidney sections from 9-month-old Pkhd1lacZ/+ mice, showing β-galactosidase expression in medullary tubules (l) and Bowman’s capsule (j). k X-Gal and eosin-stained section from a 9-month-old Pkhd1lacZ/lacZ mouse, showing cortical cysts and decreased expression of β-galactosidase in the medulla. l–m Higher magnification images of the cortex, showing decreased β-galactosidase expression in the tubular cysts and absence of expression in glomerular cysts. Bars represent 200 μm (a, d, e, h, k) and 50 μm (b, c, f, g, i, j, l, m). gl glomerulus, cy cyst
Next, we examined β-galactosidase expression in cystic kidneys from homozygous Pkhd1lacZ/lacZ mice. When the mice were 45–60 days of age, large cortical and medullary macrocysts (Fig. 3d), glomerular cysts (Fig. 3e), and mildy dilated collecting ducts (Fig. 3f) were observed amongst numerous β-galactosidase-expressing proximal tubules and collecting ducts. The glomerular cysts did not express β-galactosidase (Fig. 3e), whereas β-galactosidase expression was detected in the cells lining the mildly dilated collecting ducts and cysts (Fig. 3f,g). When the mice were 9 months of age, the expression of β-galactosidase in the Pkhd1 mutant mice had decreased in comparison with that of controls. Heterozygous Pkhd1lacZ/+ mice continued to express β-galactosidase in collecting ducts, proximal tubules, and glomerular parietal epithelial cells (Fig. 3h,j). However, 9-month-old Pkhd1lacZ/lacZ mice showed dramatically lower β-galactosidase expression in the kidney (Fig. 3k). Many of the cyst epithelial cells did not express β-galactosidase, indicating loss of Pkhd1 promoter activity at this stage (Fig. 3l,m). BUN levels were not significantly different in Pkhd1lacZ/lacZ knockout mice and wild-type mice at 3 months, 6 months, and 1 year of age (data not shown).
Ductal plate malformations and hepatic fibrosis in Pkhd1lacZ/lacZ mice
We performed histological analysis of the liver to determine the effect of loss of Pkhd1 expression on biliary duct formation. The livers from Pkhd1lacZ/lacZ mice contained numerous dilated intrahepatic bile ducts (Fig. 4c) compared with those from wild-type, age-matched controls (Fig. 4a, b). The portal veins were surrounded by multiple dilated bile ducts that were lined by a multi-layered epithelium (Fig. 4d). The hepatocytes were morphologically normal. Periportal fibrosis, one of the hallmarks of liver involvement in ARPKD, was increased in Pkhd1lacZ/lacZ mice (Fig. 4e–h). Intrahepatic bile ducts were identified by expression of cytokeratin (Fig. 4i), and hepatic arteries were identified by smooth muscle actin expression (Fig. 4j). In Pkhd1lacZ/lacZ mice, the diameters of the intrahepatic bile ducts were much greater than those of the hepatic arteries (Fig. 4k,l), exceeding the 1:1.5 ratio seen in wild-type portal tracts [18]. β−Galactosidase expression was identified in intrahepatic bile ducts of heterozygous Pkhd1lacZ/+ mice (Fig. 4m,n). In contrast to kidney cysts, Pkhd1 promoter activity was unaffected in dilated intrahepatic bile ducts of homozygous mutant mice (Fig. 4o,p). No change in β-galactosidase expression was observed in the multi-layered biliary epithelial cells lining dilated, irregular, bile ducts in Pkhd1lacZ/lacZ mice. No significant differences in serum ALT, AST, and γGT levels were identified between homozygous Pkhd1lacZ/lacZ mice and wild-type controls (data not shown).
Ductal plate malformations in Pkhd1lacZ/lacZ mice. a Hematoxylin and eosin (H&E)-stained section of the liver from a 2-month-old wild-type mouse. b High-magnification image of a central vein and portal tracts. c H&E-stained section of the liver from a 2-month-old Pkhd1lacZ/lacZ mouse, showing dilated irregular bile ducts. d Higher magnification image of a portal tract, showing multiple dilated bile ducts. e, f Trichrome-stained section of liver from an adult wild-type mouse. The arrow in f shows occasional blue staining, indicating fibrosis. g, h Trichrome-stained sections of livers from Pkhd1lacZ/lacZ mice, showing increased periportal fibrosis. i–l Sections of livers from wild-type mice (i, j) and Pkhd1lacZ/lacZ mice (k, l) stained for cytokeratin (green) and smooth muscle α-actin (red). m, n X-Gal and eosin-stained liver sections from Pkhd1lacZ/+ mice, showing β-galactosidase expression in biliary epithelial cells. o X-Gal and eosin-stained liver section from a 60-day-old Pkhd1lacZ/lacZ mouse, showing β-galactosidase expression in dilated irregular bile ducts. p Dilated intra-hepatic bile ducts in Pkhd1lacZ/lacZ mice are lined by a multi-layered epithelium. Bars represent 50 μm. ha hepatic artery, bd bile duct
Pkhd1lacZ/lacZ mice develop pancreatic and gall bladder cysts
Gross and histological abnormalities were observed in the pancreases of Pkhd1lacZ/lacZ mice. Some mice developed large pancreatic cysts (Fig. 5a,b). In addition, dilatation of extrahepatic bile ducts and gall bladder cysts were observed in 2/12 (8%) of the Pkhd1lacZ/lacZ mice that were greater than 1 year old (Fig. 5c). Histologically, the pancreata in Pkhd1lacZ/lacZ mice showed dilated intra-acinar and inter-acinar exocrine ducts with diameters greater than three-times the normal diameter when compared with those of the Pkhd1lacZ/+ heterozygotes (Fig. 5d,e). Pkhd1 promoter activity was preserved in dilated ducts, as indicated by the uniform β-galactosidase expression in the epithelial cells lining the dilated ducts. The dilated structures were identified as exocrine pancreatic ducts by immunohistochemical staining with DBA (Fig. 5f). Massive pancreatic cysts were identified in 7/69 (10%) of Pkhd1lacZ/lacZ mice.
Dilated pancreatic ducts and pancreatic and gall bladder cysts in Pkhd1lacZ/lacZ mice. a Gross pathology of the pancreas (pa) in a 2-month-old Pkhd1lacZ/lacZ mouse. b Massively cystic pancreas (pa) and normal-appearing common bile duct (cbd) and gall bladder (gb) in a 2-month-old Pkhd1lacZ/lacZ mouse. Lung (lu), liver (li), heart (ht). c Cystic gall bladder (gb) and splenomegaly (sp) in a Pkhd1lacZ/lacZ mouse. d, e X-Gal-stained sections of the pancreas from a Pkhd1lacZ/+ mouse (d) and Pkhd1lacZ/lacZ mouse (e). f DBA staining (green) and anti-smooth muscle α-actin staining (red) show dilated pancreatic ducts in a Pkhd1lacZ/lacZ mouse. Bars, 100 µm
Discussion
We deleted exons 1–3 of Pkhd1 because this genomic region contains the transcription and translation start site. Deletion of exons 1–3 and replacement with a lacZ reporter gene resulted in the formation of kidney cysts originating from the glomerulus, proximal tubule, and collecting duct. The kidney phenotype of Pkhd1lacZ/lacZ mice is distinct from the phenotypes of the Pkhd1 knockout mice that have been reported previously (Table 1). Female Pkhd1del2/del2 mutant mice in which exon 2 has been deleted develop proximal tubular dilatation but no microcysts or macrocysts [11]. Male Pkhd1del2/del2 mice have normal kidney histology. Pkhd1del3–4/del3–4 mice that lack exons 3–4 develop cysts in the collecting ducts and thick ascending limb [12]. At 9 months of age, 50% of Pkhd1del3–4/del3–4 mice had more than ten cysts per kidney, as opposed to 100% of Pkhd1lacZ/lacZ mice at the same age. No kidney cysts are seen in Pkhd1ex40 mice in which exon 40 has been deleted. The differences in the phenotypes are likely due to differences in the specific mutation of Pkhd1 rather than differences in genetic background, since the mutant mice have been analyzed after inbreeding on a C57BL/6J background (Table 1).
Pkhd1lacZ/lacZ mice develop cysts in renal collecting ducts, similar to humans with ARPKD. Cysts are also found in proximal tubules, which are sites of transient cyst formation during fetal development in human ARPKD. However, in contrast to ARPKD, which frequently presents with severe neonatal disease, Pkhd1lacZ/lacZ mice are born with morphologically normal kidneys and do not develop cysts until adulthood. This phenotype more closely resembles that of humans with late-onset ARPKD. The severity of ARPKD can be highly variable, even between individuals with the same mutation, which may reflect influences of environmental factors or modifier genes. Pkhd1lacZ/lacZ mice develop glomerular cysts, a finding not previously reported in ARPKD and absent in the other Pkhd1 knock-out models. Studies using Pkhd1lacZ/+ mice indicate that Pkhd1 is expressed in Bowman’s capsule. Hiesberger et al. recently showed that the expression of Pkhd1 is regulated by the transcription factor hepatocyte nuclear factor (HNF)-1β [8]. Kidney-specific inactivation of HNF-1β in transgenic mice results in decreased expression of Pkhd1 and formation of renal cysts [16]. Mutations of HNF-1β in humans are associated with glomerulocystic kidney disease. Taken together, these observations suggest that the formation of glomerular cysts in humans with HNF-1β mutations may be due to decreased expression of Pkhd1.
Since the Pkhd1lacZ/lacZ mice carry a lacZ reporter gene replacing exons 1–3, the expression of β-galactosidase reflects Pkhd1 promoter activity. Heterozygous Pkhd1lacZ/+ mice exhibit β-galactosidase expression in epithelial cells in the kidney, liver, and pancreas, consistent with the known pattern of expression of Pkhd1. Pkhd1 promoter activity is preserved in cystic and non-cystic tubules in young homozygous Pkhd1lacZ/lacZ mice. In older mutant animals, Pkhd1 promoter activity is diminished, and only a minority of cyst epithelial cells expresses β-galactosidase. Moreover, expression is also decreased in non-cystic tubules in the renal medulla. This observation suggests that the expression of Pkhd1 is controlled by a transcriptional autoregulatory mechanism.
Pkhd1lacZ/lacZ mice exhibit progressive cyst formation that is manifested after the completion of postnatal nephrogenesis. This observation suggests that Pkhd1 is not essential for tubulogenesis but is required for the maintenance of normal tubule architecture. Previous studies have shown that the Pkhd1 gene product, fibrocystin, interacts in a complex with polycystin-2, and the two proteins together regulate flow-induced intracellular calcium release [9, 19]. Fibrocystin, in turn, undergoes calcium-dependent proteolytic cleavage and translocates to the nucleus, where it may participate in gene transcription [20]. Kidneys from homozygous Pkhd1lacZ/lacZ mice have diminished expression of Pkd1 and Pkd2. This alteration in gene expression is not seen in kidneys from Pkhd1del3–4/del3–4 mice; however, mice carrying mutations of both Pkhd1 and Pkd1 exhibit more severe cystic kidney disease than mice with mutations of Pkhd1 by itself [12]. Taken together, these findings suggest the existence of a transcriptional relationship between Pkhd1, Pkd1, and Pkd2.
Dilated, irregular, intrahepatic bile ducts are consistently seen in homozygous mutant Pkhd1lacZ/lacZ mice. This lesion resembles the classical ductal plate malformation seen in humans with ARPKD. A similar hepatic phenotype has been observed in Pkhd1del3–4/del3–4, Pkhd1ex40and Pkhd1del2/del2 mice. Pkhd1lacZ/lacZ mice, Pkhd1del2/del2 mice, and Pkhd1del3–4/del3–4 mice also develop cysts in the exocrine ducts of the pancreas. Pancreatic duct dilatation and cysts have not been reported in ARPKD, although fibrosis of the pancreas has been described. Pancreatic disease was not observed in the Pkhd1ex40 mouse, which may reflect the persistent expression of a fibrocystin mutant lacking 63 amino acids, whereas Pkhd1lacZ is a null allele.
References
Igarashi P, Somlo S (2002) Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol 13:2384–2398
Adeva M, El-Youssef M, Rossetti S, Kamath PS, Kubly V, Consugar MB, Milliner DM, King BF, Torres VE, Harris PC (2006) Clinical and molecular characterization defines a broadened spectrum of autosomal recessive polycystic kidney disease (ARPKD). Medicine (Baltimore) 85:1–21
Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M, Milliner DS, Torres VE, Harris PC (2002) The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet 30:259–269
Nagasawa Y, Matthiesen S, Onuchic LF, Hou X, Bergmann C, Esquivel E, Senderek J, Ren Z, Zeltner R, Furu L, Avner E, Moser M, Somlo S, Guay-Woodford L, Buttner R, Zerres K, Germino GG (2002) Identification and characterization of Pkhd1, the mouse orthologue of the human ARPKD gene. J Am Soc Nephrol 13:2246–2258
Bergmann C, Senderek J, Sedlacek B, Pegiazoglou I, Puglia P, Eggermann T, Rudnik-Schoneborn S, Furu L, Onuchic LF, De Baca M, Germino GG, Guay-Woodford L, Somlo S, Moser M, Buttner R, Zerres K (2003) Spectrum of mutations in the gene for autosomal recessive polycystic kidney disease (ARPKD/PKHD1). J Am Soc Nephrol 14:76–89
Ward CJ, Yuan D, Masyuk TV, Wang X, Punyashthiti R, Whelan S, Bacallao R, Torra R, LaRusso NF, Torres VE, Harris PC (2003) Cellular and subcellular localization of the ARPKD protein; fibrocystin is expressed on primary cilia. Hum Mol Genet 12:2703–2710
Kaimori JY, Nagasawa Y, Menezes LF, Garcia-Gonzalez MA, Deng J, Imai E, Onuchic LF, Guay-Woodford LM, Germino GG (2007) Polyductin undergoes notch-like processing and regulated release from primary cilia. Hum Mol Genet 16:942–956
Hiesberger T, Shao X, Gourley E, Reimann A, Pontoglio M, Igarashi P (2005) Role of the hepatocyte nuclear factor-1beta (HNF-1beta) C-terminal domain in Pkhd1 (ARPKD) gene transcription and renal cystogenesis. J Biol Chem 280:10578–10586
Wang S, Zhang J, Nauli SM, Li X, Starremans PG, Luo Y, Roberts KA, Zhou J (2007) Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol Cell Biol 27:3241–3252
Moser M, Matthiesen S, Kirfel J, Schorle H, Bergmann C, Senderek J, Rudnik-Schoneborn S, Zerres K, Buettner R (2005) A mouse model for cystic biliary dysgenesis in autosomal recessive polycystic kidney disease (ARPKD). Hepatology 41:1113–1121
Woollard JR, Punyashtiti R, Richardson S, Masyuk TV, Whelan S, Huang BQ, Lager DJ, vanDeursen J, Torres VE, Gattone VH, LaRusso NF, Harris PC, Ward CJ (2007) A mouse model of autosomal recessive polycystic kidney disease with biliary duct and proximal tubule dilatation. Kidney Int 72:328–336
Garcia-Gonzalez MA, Menezes LF, Piontek KB, Kaimori J, Huso DL, Watnick T, Onuchic LF, Guay-Woodford LM, Germino GG (2007) Genetic interaction studies link autosomal dominant and recessive polycystic kidney disease in a common pathway. Hum Mol Genet:16:1940–1950
Kapur RP, Hoyle GW, Mercer EH, Brinster RL, Palmiter RD (1991) Some neuronal cell populations express human dopamine [beta]-hydroxylase-lacZ transgenes transiently during embryonic development. Neuron 7:717–727
Wang Y, Spatz MK, Kannan K, Hayk H, Avivi A, Gorivodsky M, Pines M, Yayon A, Lonai P, Givol D (1999) A mouse model for achondroplasia produced by targeting fibroblast growth factor receptor 3. Proc Natl Acad Sci U S A 96:4455–4460
Igarashi P, Shashikant CS, Thomson RB, Whyte DA, Liu-Chen S, Ruddle FH, Aronson PS (1999) Ksp-cadherin gene promoter. II. Kidney-specific activity in transgenic mice. Am J Physiol Renal Physiol 277:F599–F610
Hiesberger T, Bai Y, Shao X, McNally BT, Sinclair AM, Tian X, Somlo S, Igarashi P (2004) Mutation of hepatocyte nuclear factor-1beta inhibits Pkhd1 gene expression and produces renal cysts in mice. J Clin Invest 113:814–825
Wu G, Tian X, Nishimura S, Markowitz GS, D'Agati V, Hoon Park J, Yao L, Li L, Geng L, Zhao H, Edelmann W, Somlo S (2002) Trans-heterozygous Pkd1 and Pkd2 mutations modify expression of polycystic kidney disease. Hum Mol Genet 11:1845–1854
Crawford AR, Lin XZ, Crawford JM (1998) The normal adult human liver biopsy: a quantitative reference standard. Hepatology 28:323–331
Wu Y, Dai X-Q, Li Q, Chen CX, Mai W, Hussain Z, Long W, Montalbetti N, Li G, Glynne R, Wang S, Cantiello HF, Wu G, Chen X-Z (2006) Kinesin-2 mediates physical and functional interactions between polycystin-2 and fibrocystin. Hum Mol Genet 15:3280–3292
Hiesberger T, Gourley E, Erickson A, Koulen P, Ward CJ, Masyuk TV, Larusso NF, Harris PC, Igarashi P (2006) Proteolytic cleavage and nuclear translocation of fibrocystin is regulated by intracellular Ca2+ and activation of protein kinase C. J Biol Chem 281:34357–34364
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants R01DK42921 and R01DK67565 and the UT Southwestern O’Brien Kidney Research Core Center (NIH P30DK079328).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Williams, S.S., Cobo-Stark, P., James, L.R. et al. Kidney cysts, pancreatic cysts, and biliary disease in a mouse model of autosomal recessive polycystic kidney disease. Pediatr Nephrol 23, 733–741 (2008). https://doi.org/10.1007/s00467-007-0735-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-007-0735-4