Abstract
Renal scarring is known to be associated with hypertension. The primary objective of this study was to investigate the prevalence of renal scarring in children referred to our clinic with hypertension. The secondary objective was to compare renal ultrasound (US) examination with dimercaptosuccinic acid (DMSA) renal scan in diagnosing renal scars in these patients. The study included 159 patients who underwent DMSA renal scan as well as renal US for the evaluation of hypertension of unknown etiology. Thirty-three (21%) patients were found to have renal scars; their demographic details, including mean age and gender distribution, were not significantly different from those without renal scars. In comparison with the DMSA renal scan, sensitivity and specificity of renal US in diagnosing renal scars were 36% and 94%, respectively. In our study, in which the prevalence of scarring was 21%, this gave positive predictive and negative predictive values of 63% and 85%, respectively. In conclusion, our study indicates that renal scarring is present in 21% of otherwise healthy children who are evaluated for newly diagnosed hypertension, and renal US is not a sensitive imaging modality to rule out renal scarring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence and incidence of hypertension in children is lower than adults, but in the last decade, the prevalence has increased from 1% to 5% [1]. Unlike in most adults, hypertension in children, particularly in the younger age group, is usually secondary in origin. The most common cause of secondary hypertension in children is renovascular or renal parenchymal disease, which account for about 60–70% of pediatric cases with hypertension [2–4]. Renal scarring, which is a well-known cause for secondary hypertension, is commonly attributed to one or more episodes of acute pyelonephritis in the presence of vesicoureteral reflux (VUR) in younger children [5]. The current gold standard for diagnosing renal scars is the dimercaptosuccinic acid (DMSA) renal scan [6, 7]. There is no existing data on the prevalence of renal scars in pediatric patients with hypertension. The primary objective of our study was to investigate the prevalence of renal scarring in pediatric patients referred to our clinic with hypertension. The secondary objective was to compare renal ultrasound (US) examination with DMSA renal scan in the diagnosis of renal scars.
Patients and methods
This retrospective study included patients with arterial hypertension in the age group of 1 month to 18 years who were referred to our general nephrology or hypertension clinic between 2000 and 2005. Blood pressure (BP) was measured as per the recommendations of the National High Blood Pressure Education Program Working Group’s Third Task Force [8], and hypertension was defined as BP more than the 95th percentile for age, gender, and height on more than three occasions. Patients who were found to have congenital renal abnormalities, decrease in renal function as diagnosed by estimated glomerular filtration rate (GFR) by Schwartz formula [9] of less than 90 ml/min per 1.73 m2, or hematuria and/or proteinuria were excluded. Using these criteria, 172 patients with hypertension who had DMSA renal scan as well as renal US examination were identified. In 72 (42%) patients, the DMSA renal scans had been reported as abnormal. To eliminate interobserver variability, all available “abnormal” DMSA scans (59 of 72) were reanalyzed by a single radiologist. Those remaining 13 patients whose DMSA scans were unavailable for reanalysis were excluded. Data on the remaining 159 patients was analyzed.
Of the 59 patients initially reported as having abnormal DMSA scans, 33 (56%) were diagnosed as abnormal on the second review. The DMSA scans on the remaining 26 (44%) patients were diagnosed as normal on the second review, and for analysis purposes, they were included in the group with normal DMSA scans. Therefore, 33 patients with abnormal DMSA scans were compared with 126 patients with normal DMSA scans; all 159 patients also had renal US examination. Renal US examinations for the 33 patients with abnormal DMSA scans were reviewed again by the same radiologist. The renal US was classified as abnormal if the location of the sonographic defect, characterized by abnormal echogenicity or size, exactly matched the location of scarring on DMSA scan. Renal scarring on the 33 abnormal DMSA renal scans were graded according to the classification of the International Reflux Study by Peipz et al., which is as follows [10]:
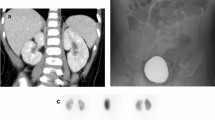
-
Type 1:
Intact outline, reniform shape of normal size but with a large polar area showing photon deficiency
-
Type 2:
Peripheral focal defects in a nondeformed kidney of similar size to the contralateral kidney
-
Type 3:
Normal shape, smaller than the opposite kidney, with a proportionate uptake by the affected kidney of < 45% if the contralateral kidney was normal
-
Type 4a:
Distorted image of normal-sized kidney with peripheral photon absent areas and corresponding loss of renal contour
-
Type 4b:
Appearance of type 4a but small kidney
Renal US examinations were performed by trained technicians using Acuson Sequoia Machines manufactured by Siemens, with frequency of probes ranging between 2.5 MHz and 15 MHz depending on patient size. Longitudinal and transverse gray-scale images were obtained ventrally and dorsally for all kidneys. All kidneys were assessed for size (comparison with standards for age and weight), echogenicity, corticomedullary differentiation, and cortical outline. All DMSA renal scans were performed by pediatric nuclear medicine technicians using technetium dimercaptosuccinic acid (Tc-99 m DMSA) with either two- or three-headed gamma cameras and single photon emission computed tomography (SPECT). Data obtained were analyzed using SPSS version 13 software. Comparison between patients with normal and abnormal DMSA scans was done using chi-square test. A p value of less than 0.05 was considered to be significant.
Results
The age of 33 patients with abnormal DMSA renal scans ranged from 1 month to 17 years, with a median age of 11 years. Included were 18 (55%) females and 15 (45%) males. Table 1 details the demographic data of the patients with abnormal DMSA scan (n = 33), and a comparison of these patients with 126 patients with normal DMSA scans revealed no significant difference in age, gender, ethnicity, or body mass index (BMI) percentiles.
Of the 159 patients included in the study, 140 (88%) had normal renal US examination, whereas it was suggestive of renal scarring (abnormal) in 19 (12%) patients. Of those patients with abnormal renal US examinations, 12 (63%) had abnormal and seven (37%) had normal DMSA renal scans (Table 2). Also, of the 140 patients who had normal ultrasounds, DMSA scan was found to be abnormal in 21 (15%). Sensitivity and specificity of US in diagnosing renal scars in our patient population were 36% and 94%, respectively. The corresponding positive (PPV) and negative predictive values (NPV) for US were 63% and 85%, respectively.
Among the 33 patients with abnormal DMSA renal scans, 22 had unilateral scarring and 11 had bilateral scarring. Of the 44 kidney units with scarring (Table 3), 35 (79%) were classified as having type 2 or higher scarring. Twenty-three (52%) scarred kidneys were seen in patients older than 12 years, and included were 11 (25%) kidney units with type 4 scarring. Only five of the 33 patients (15%) with abnormal DMSA scans had a past history of urinary tract infection (UTI). Of the patients with abnormal DMSA scans, voiding cystourethrogram (VCUG) was done in 20 patients, six (30%) of whom showed presence of VUR.
Discussion
Evaluation of hypertension in otherwise healthy children is directed mostly at the diagnosis of an underlying treatable cause for secondary hypertension [11]. Renal scarring is a well-known cause for hypertension and proteinuria, with progression to end-stage renal disease (ESRD) in some pediatric patients [12, 13]. The 24-h ambulatory blood pressure monitoring (ABPM) is being increasingly used in children with renal disease, including those with scarring [14]. In a study published by Milosevski et al., ABPM demonstrated nocturnal systolic hypertension in all patients with renal scarring [15]. Patzer et al. demonstrated that BP readings measured by ABPM correlated with degree of renal scarring in their patients [16]. In another study, Silva et al. assessed the risk of hypertension in children with primary VUR after a median follow-up period of 72 months [17]. Renal damage was present in 318 (48%) of 664 patients. The prevalence of hypertension increased with age from 1.7% for younger age groups to 35% in patients >20 years at end of follow-up. The probability of hypertension at 21 years was estimated to be 0% for patients without renal damage, 15% for patients with unilateral renal damage, and 45% for patients with bilateral renal damage. Furthermore, it was estimated by survival analysis that 50% of patients with unilateral and bilateral renal damage would have sustained hypertension at about 30 and 22 years of age, respectively. Interestingly, despite such observations, the prevalence of renal scarring in pediatric patients undergoing routine evaluation for newly diagnosed hypertension is not known.
Pediatric patients referred to specialty clinics undergo numerous investigations, including renal US examination, to look for any obvious abnormality, including differences in renal size. However, renal US is not a good imaging modality for detecting renal scars, even though it is not uncommon to see reports indicating the possibility of a renal scar on US examination. DMSA renal scan is currently the gold standard for diagnosing renal scars [6, 7, 18]. Moorthy et al. reported that although US had a good specificity for detecting renal scarring, it had a low sensitivity and could not be substituted for DMSA renal scan in diagnosing renal scars [19]. Similar results were reported by Temiz et al., where the authors concluded that US examination was an inappropriate study for diagnosing renal scars in children with primary VUR, irrespective of the grade of reflux [20]. In our patient population, the renal US examination revealed a very low sensitivity of 36%, a specificity of 94%, low PPV value of 63%, and NPV of 85%. Of the 59 patients who had DMSA renal scan, 26 (44%) were interpreted as normal on the second review, which highlights the limitations in interpreting questionable renal scars on DMSA renal scans and the potential impact of the interobserver variability. Our study did not include patients with proteinuria, hematuria, or reduced GFR, and yet 33 (56%) patients had definite renal scars on DMSA renal scans, indicating that the incidence of renal scarring in children presenting with hypertension may in fact be higher than reported in our study.
The patients included in our study were referred to our general nephrology or hypertension clinic for the evaluation of hypertension, and all were without any preexisting renal or any other comorbid pathology. All patients underwent extensive workup to rule out secondary hypertension, and all had renal US examination as well as the DMSA renal scan. One weakness of this study was its retrospective nature. This led to the difficulty in retrieving 13 DMSA scans, thus prohibiting their reanalysis and possibly altering our outcome. Nevertheless, one important feature of our study that may have increased the strength of our findings was to eliminate bias due to interobserver variability. All radiological studies were reviewed by a single radiologist, and patients with doubtful imaging results were excluded from the study. The results of our study show that of the 159 patients evaluated for hypertension, 33 (21%) had renal scarring. Our study also showed that out of the 44 kidney units evaluated, 35 (79%) had type two or higher scarring. Interestingly, in patients older than 12 years, of the 23 kidney units, 11 (48%) had type 4 renal scarring. This is of particular importance because older children generally undergo fewer investigations due to a higher incidence of primary hypertension. Recognition of renal scarring in this age group is important because of its potential for the progression of renal disease. Of the patients with scarring, only five had a history of UTI as reported by the parent. The possibility of asymptomatic or undiagnosed UTI in some patients could not be ruled out. VCUG was positive in six of the 20 patients who had a VCUG done. These observations indicate that the scarring may have been a result of reflux nephropathy, even though in some of them the VUR had resolved, or the scars may have been congenital in origin, as may occur with renal hypodysplasia [21].
In conclusion, our study revealed that renal scarring was present in 21% of otherwise healthy children who were evaluated for newly diagnosed hypertension and that renal US examination was not a sensitive imaging modality in diagnosing renal scars in such patients. The identification of renal scars not only helped diagnose more patients with secondary hypertension, it also helped us take appropriate measures to prevent the progression of the renal disease in some patients.
References
Sorof JM, Lai D, Turner J, Poffenbarger T, Portman RJ (2004) Overweight, ethnicity, and the prevalence of hypertension in school-aged children. Pediatrics 113:475–482
Arar MY, Hogg RJ, Arant BS Jr, Seikaly MG (1994) Etiology of sustained hypertension in children in the southwestern United States. Pediatr Nephrol 8:186–189
Falkner B, Sadowski RH (1995) Hypertension in children and adolescents. Am J Hypertens 8:106–110
Hanna JD, Chan JC, Gill JR Jr (1991) Hypertension and the kidney. J Pediatr 118:327–340
Lahdes-Vasama T, Niskanen K, Ronnholm K (2006) Outcome of kidneys in patients treated for vesicoureteral reflux (VUR) during childhood. Nephrol Dial Transplant 21:2491–2497
Bhatnagar V, Mitra DK, Agarwala S, Kumar R, Patel C, Malhotra AK, Gupta AK (2002) The role of DMSA scans in evaluation of the correlation between urinary tract infection, vesicoureteric reflux, and renal scarring. Pediatr Surg Int 18:128–134
Smellie JM, Shaw PJ, Prescod NP, Bantock HM (1988) 99mTc dimercaptosuccinic acid (DMSA) scan in patients with established radiological renal scarring. Arch Dis Child 63:1315–1319
National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents (1996) Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: a working group report from the National High Blood Pressure Education Program. Pediatrics 98:649–658
Schwartz GJ, Brion LP, Spitzer A (1987) The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 34:571–590
Piepsz A, Tamminen-Mobius T, Reiners C, Heikkilä J, Kivisaari A, Nilsson NJ, Sixt R, Risdon RA, Smellie JM, Söderborg B (1998) Five-year study of medical or surgical treatment in children with severe vesico-ureteral reflux dimercaptosuccinic acid findings. International Reflux Study Group in Europe. Eur J Pediatr 157:753–758
Mitsnefes MM (2006) Hypertension in children and adolescents. Pediatr Clin North Am 53:493–512, viii
Soergel M, Schaefer F (2002) Effect of hypertension on the progression of chronic renal failure in children. Am J Hypertens 15:53S–56S
Vachvanichsanong P (2007) Urinary tract infection: one lingering effect of childhood kidney diseases–review of the literature. J Nephrol 20:21–28
Graves JW, Althaf MM (2006) Utility of ambulatory blood pressure monitoring in children and adolescents. Pediatr Nephrol 21:1640–1652
Milosevski G, Kostic M, Babic D, Jovanović O, Kruscić D, Stanić M, Peco-Antić A (2005) Classic and automated blood pressure monitoring in children with scarring nephropathy. Srp Arh Celok Lek 133:417–423
Patzer L, Seeman T, Luck C, Wuhl E, Janda J, Misselwitz J (2003) Day- and night-time blood pressure elevation in children with higher grades of renal scarring. J Pediatr 142:117–122
Simoes e Silva AC, Silva JM, Diniz JS, Pinheiro SV, Lima EM, Vasconcelos MA, Pimenta MR, Oliveira EA (2007) Risk of hypertension in primary vesicoureteral reflux. Pediatr Nephrol 22:459–462
Rushton HG, Majd M, Jantausch B, Wiedermann BL, Belman AB (1992) Renal scarring following reflux and nonreflux pyelonephritis in children: evaluation with 99mtechnetium-dimercaptosuccinic acid scintigraphy. J Urol 147:1327–1332
Moorthy I, Wheat D, Gordon I (2004) Ultrasonography in the evaluation of renal scarring using DMSA scan as the gold standard. Pediatr Nephrol 19:153–156
Temiz Y, Tarcan T, Onol FF, Alpay H, Simsek F (2006) The efficacy of Tc99m dimercaptosuccinic acid (Tc-DMSA) scintigraphy and ultrasonography in detecting renal scars in children with primary vesicoureteral reflux (VUR). Int Urol Nephrol 38:149–152
Marra G, Oppezzo C, Ardissino G, Dacco V, Testa S, Avolio L, Taioli E, Sereni F; ItalKid Project (2004) Severe vesicoureteral reflux and chronic renal failure: a condition peculiar to male gender? Data from the ItalKid Project. J Pediatr 144:677–681
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmed, M., Eggleston, D., Kapur, G. et al. Dimercaptosuccinic acid (DMSA) renal scan in the evaluation of hypertension in children. Pediatr Nephrol 23, 435–438 (2008). https://doi.org/10.1007/s00467-007-0656-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-007-0656-2