Abstract
To assess renal inflammation and its sequelae in Kawasaki disease (KD) patients, we conducted a prospective study in a university medical center setting in Taiwan. From June 2002 to January 2005, 50 children with KD were enrolled, and after admission, all received technetium-99m dimercaptosuccinic acid scintigraphy single photon emission computed tomography (DMSA renal SPECT), the results of which were used as the reference standard for determining renal inflammation. Patients with renal inflammation underwent another DMSA renal SPECT more than 6 months later to evaluate the sequelae. We found that 26 of the 50 patients (52%) had renal inflammatory foci. There were no significant relationships between clinical or laboratory parameters and renal involvement in KD, except the presence of coronary artery lesions [P < 0.01; odds ratio (OR) 5.18; 95% confidence interval (CI) 1.52–17.65]. Although all patients were free of clinical symptoms, the 6-month follow-up DMSA renal SPECT showed renal scarring in 11 of the 24 patients (46%). Patients with an initial abnormal renal ultrasound did predict a greatly increased risk of scarring (P < 0.05; OR 16.2; 95% CI 1.27–206.20). In conclusion, this study demonstrated that the potential long-term clinical impact of KD is not limited to coronary artery lesion sequelae but also includes renal scar formation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Kawasaki disease (KD) (mucocutaneous lymph node syndrome) is an infants’ and children’s illness characterized by irritability, fever, rash, swollen hands and feet, conjunctivitis, swollen lymph glands in the neck, and irritation and inflammation of the mouth, lips, and throat [1–3]. The most common pathologic findings are systemic vasculitis and a high incidence of inflammatory lesions affecting various organs [4, 5]. The most serious and life-threatening aspect of KD is the development of angitis and aneurysmal dilation of the coronary arteries accompanied by thrombosis and, in some cases, death [2, 3, 6, 7].
The involvement of the genitourinary tract in KD manifests itself clinically in various forms. The most common presentations are sterile pyuria and proteinuria; fewer patients have transient microscopic hematuria [2, 3, 8, 9]. Although they are rare, severe complications such as renovascular hypertension, hemolytic uremic syndrome, interstitial nephritis, and acute renal failure have been reported [10–14]. Vascular involvement occurs in blood vessels of small and medium size, such as renal arteries; renal involvement is thus possible [15]. Whether renal inflammation is associated with KD has not been systematically evaluated.
Evaluating renal inflammation has traditionally depended on clinical manifestations, urography, and ultrasonography; more effective options are now available. Technetium-99m dimercaptosuccinic acid (DMSA) scintigraphy is a highly sensitive and specific noninvasive imaging modality for detecting renal inflammation that can demonstrate radiotracer uptake defects in acute renal inflammatory foci [16]. The application of single photon emission computed tomography (SPECT) together with DMSA scintigraphy (DMSA renal SPECT) increases to 96% the sensitivity of DMSA in detecting inflammatory foci [17]. Furthermore, DMSA scintigraphy is more effective and sensitive than urography and ultrasonography in detecting permanent renal damage (i.e., scarring) [18, 19].
The purpose of this study was to assess renal inflammation and its sequelae in patients with childhood KD. We wanted to (1) evaluate the incidence of renal inflammation using DMSA renal SPECT, (2) investigate the associated risks resulting from renal involvement, and (3) discuss the development of renal sequelae and its clinical implications.
Methods
Patient selection
Children admitted to our hospital, a university medical center in Taiwan, with acute febrile KD were considered eligible for this prospective study. KD was defined according to standard clinical diagnostic criteria [20]. The exclusion criteria were (a) a previous history of urinary tract infection, (b) coincident congenital urogenital abnormality or uropathy, (c) a urinary tract infection between the initial and follow-up scintigram, (d) a space-occupying lesion at renal ultrasound, and (e) positive results from urine culture. When the patient’s fever had subsided for 48 h, the patient was discharged from the hospital and followed up in the outpatient clinic. The study protocol was approved by our hospital’s institutional review board, and written informed consent was obtained from the patients’ parents.
Data collection
The protocol for treatment of patients diagnosed with KD was based on the recommendations of the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the American Heart Association’s Council on Cardiovascular Diseases in the Young [21]. Patients were given intravenous immunoglobulin (2 gm/kg) and aspirin (80–100 mg/kg day; orally) but no antibiotics. When patients were afebrile, they were given only 3–5 mg/kg of aspirin (orally). Lab tests included a complete blood count with a differential count, C-reactive protein, and erythrocyte sedimentation rate. Liver enzymes and renal function were assessed upon or soon after admission. Liver enzymes were considered abnormal if alanine aminotransferase was > 45 U/l. Renal function was defined as abnormal if creatinine was > 0.4 mg/dl in infants (≤ 12 months old) or > 0.7 mg/dl in children (> 12 months old) [22]. Pyuria was considered present if a urinary analysis showed a white blood cell count of ≥ 10/high power field; hematuria, if the red blood cell count was ≥ 5/high power field; and proteinuria, if the protein level was > 100 mg/dl. At the same time, a urine culture was done to rule out the possibility of a urinary tract infection.
An initial echocardiographic examination (SONOS 5500; Hewlett-Packard, Les Ullis, France) using a broad-band transducer (S8 transducer: 3–8 MHz, or S12 transducer: 5–12 MHz) to evaluate coronary artery abnormalities was done by a trained pediatric cardiologist on all patients entering the study. Renal ultrasound (SSD-1200; Aloka America, Wallingford, CT, USA) with a convex 5-MHz probe in B mode was done by a trained pediatric nephrologist upon admission to exclude congenital uropathy and space-occupying lesions. The results of a renal ultrasound were classified as abnormal if one of the following features was observed: parenchymal hyperechogenicity, a focal lesion with hyper- or hypoechogenicity, thickening of the renal pelvis wall, corticomedullary differentiation, or significant enlargement of the kidney length or width compared with the opposite kidney and compared with the normal range for a patient’s age. A repeat renal ultrasound was also done at the 6-month follow-up visit only if the initial DMSA renal SPECT was positive.
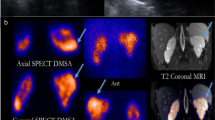
An initial DMSA renal SPECT was done within 1 week of hospitalization in the manner previously reported [23, 24]. The results were used as the reference standard for determining renal inflammation. Acute inflammation was indicated if the image showed either diffuse diminished DMSA uptake or focal areas of decreased cortical DMSA uptake. In general, a renal inflammatory lesion takes at least 5 months to stabilize [25]. Patients with positive renal inflammation underwent a second DMSA renal SPECT at least 6 months later, and, depending upon the results, the patients were placed in the “scar group” or the “nonscar group.” A renal scar was diagnosed when the follow-up image showed a defect in the renal outline located in a previously identified inflammatory focus.
The first clinical follow-up occurred within 2 weeks of discharge. Follow-up echocardiographic examinations were done at this visit and at 6 weeks and 6 months after discharge in all patients. Because most coronary artery lesions were not apparent during the initial diagnosis, patients were considered positive for lesions if the internal diameter of the coronary artery was > 3 mm in children younger than 5 years or > 4 mm in children at least 5 years old at the 2-week follow-up echocardiography [26]. When a coronary artery was larger than normal (i.e., dilated) and did not have a segmental aneurysm, we made a further diagnosis of ectatic coronary disease. Segmental coronary aneurysms were classified as small (3- to 5-mm internal diameter), medium (5- to 8-mm internal diameter), or giant (> 8-mm internal diameter) [6, 21]. A persistent coronary artery lesion was identified if the lesion had not regressed to normal by the 6-month follow-up visit.
Statistical methods
Continuous data were expressed as mean ± standard error, and differences between groups were analyzed using the Mann-Whitney U test. The chi-squared test corrected for continuity was used to compare frequencies. For < 5 cases, we used Fisher’s exact test. Statistical significance was set at P < 0.05. Using univariate analysis, an odds ratio (OR), 95% confidence interval (95% CI), and statistical associations were then calculated to estimate the risk of renal inflammation and renal scarring in relation to the parameters from laboratory and image investigations. All statistical analyses were performed using a commercial software program (STATA 7; Stata Corp., College Station, TX, USA).
Results
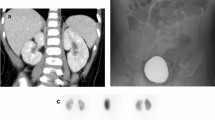
From June 2002 to January 2005, 50 patients (33 boys and 17 girls; age range, 3–89 months; mean, 20.2 ± 2.7 months; median, 13 months) were enrolled consecutively in this study. All patients had leukocytosis (mean, 17,081 ± 899/mm3), with a marked erythrocyte sedimentation rate (mean, 67.7 ± 4.3 mm/hr) and elevated C-reactive protein (mean, 103.3 ± 8.1 mg/l). Twenty-six (52%) had elevated alanine aminotransferase and aspartate aminotransferase, 19 (38%) had pyuria, six (12%) had hematuria, and six had proteinuria. Most patients had normal serum urea nitrogen (mean, 9.2 ± 3.7), and serum creatinine (mean, 0.4 ± 0.1), but two patients had mild elevated creatinine levels (0.6 mg/dl in a 5-month-old male infant; 0.8 mg/dl in a 12-month-old boy). The initial renal ultrasound revealed increased cortical echogenicity, enlarged kidneys, or increased corticomedullary differentiation in five patients (10%). Echocardiography showed that 20 patients had acute-stage coronary artery lesions: 10 (50%) had ectatic coronary disease, seven (35%) had a small aneurysm, two (10%) had a medium aneurysm, and one had a giant aneurysm. The initial DMSA renal SPECT showed that 26 of the 50 patients had renal inflammatory foci, typically several small focal areas of decreased DMSA uptake scattered in the kidneys (Fig. 1a).
Based on the initial renal scan findings, the clinical characteristics, laboratory findings, and image studies of patients with (abnormal-DMSA group) or without (normal-DMSA group) renal inflammation were compared (Table 1). In brief, there were no significant associations between clinical or laboratory parameters and renal involvement in KD, except for the presence of coronary artery lesions (P < 0.05). A univariate analysis of the risk factors resulting from renal inflammation showed that a coronary artery lesion increased the risk of renal inflammation in patients with KD (OR 5.18; 95% CI 1.52–17.65) (Table 2). Though the sensitivity for predicting renal inflammation from coronary artery lesions revealed via echocardiography was only 57.7% (15/26), the specificity was 79.2% (19/24), the positive predictive value was 75% (15/20), and the negative predictive value was 63.3% (19/30).
The clinical course in all 50 patients was uneventful. No hypertensive episodes occurred during the admission period. In brief, the results of the follow-up DMSA renal SPECT showed that 11 of the 24 patients (two patients missed the follow-up scan) (46%) had renal scarring caused by renal inflammation (Fig. 1b). In addition, echocardiograms revealed persistent coronary aneurysms in four patients: two with renal scarring, one without renal scarring, and one who had not received a follow-up DMSA renal SPECT because of a negative initial scan. Only one patient had an abnormal follow-up renal ultrasound, a patient who also had an abnormal follow-up echocardiogram.
Patients with an initial abnormal renal ultrasound showed a greatly increased risk of scarring (OR 16.2; 95% CI 1.27-206.20) (Table 2). Though the sensitivity of the initial abnormal renal ultrasound in predicting renal scarring was low (36.4%, 4/11), the specificity was 100% (13/13); the positive predictive value was 100% (4/4), and the negative predictive value was 65.0% (13/20).
Discussion
Renal involvement in childhood KD is usually clinically silent, that is, it is generally mild and lacks obvious clinical signs or symptoms; therefore, it has generally been considered as urethritis or meatitis [7]. The present study, which found surprising evidence of renal inflammation, might explain why renal involvement is not easily detected and why clinicians have overlooked its significance in KD. In our series, only 19 of 50 patients presented with pyuria, six patients presented with hematuria, six patients presented with proteinuria, and two patients presented with mildly elevated creatinine level. None had abnormal levels of serum urea nitrogen or presented with edema or oliguria, which are related to symptoms of renal involvement.
Our results are consistent with several reports of clinically significant renal impairment in KD [10–14]; renal pathology has also been described on both biopsy and postmortem examination. Histological findings included a normal glomerulus or mild expansion of the mesangial matrix; interstitial infiltration of lymphocytes, plasmocytes, and eosinophils; and a focus of tubular necrosis [12–14, 27]. In a series of histopathological examinations from 25 patients who died of KD, four infants had significant renal vascular involvement, including panarteritis of the interlobar and smaller arteries, arteriolitis, phlebitis, thromboarteritis, and intimal proliferative occlusive arteritis [28]. There is also laboratory evidence supporting the hypothesis that renal involvement in childhood KD is quite common. One study [29] reported that patients with KD had infiltration of immunoglobulin (Ig) A plasma cells systemically spread to vascular tissue and kidney parenchyma. Another [30] showed antiendothelial antibody deposits in the renal mesangium. One very recent study [31] investigated the hyponatremia in KD patients and suspected some KD patients may have significant renal parenchymal injury. However, we did not have sufficient data to confirm this finding based on our initial study design. Still, two other studies [32, 33] showed evidence that levels of urinary cytokines (interleukin 6 and 8) were consistently elevated in patients with acute childhood KD. No association was evident between urinary cytokine levels and urinary abnormalities such as pyuria and hematuria [33]. The authors therefore suggested the presence of an inflammatory process within the renal parenchyma in most patients [32]. We also found that renal inflammation was not significantly correlated with pyuria or hematuria. But whether the renal inflammation that we found in KD was attributable solely to renal vasculitis or, rather, to other immune-mediated mechanisms awaits further investigation.
Renal ultrasound is the preferred and most widely available tool to detect renal disease. Only one study [9], however, has reported using renal sonography in KD: it found increased cortical echogenicity, enlarged kidneys, and increased corticomedullary differentiation, leading the authors to conjecture that vasculitis of the kidneys, with its resultant fibrinoid deposits and cellular infiltrations, leads to ischemia. Our study had similar findings, but the incidence of abnormal renal ultrasound was only 10%. Therefore, to detect acute renal injury and chronic renal sequelae in children with KD, we used DMSA renal SPECT because of its high sensitivity and specific imaging capability [16–19]. Fifty-two percent of our patients had renal involvement during the acute stage of KD. Only coronary artery lesions significantly increased the risk of renal vascular involvement in KD children, but they were not significant for predicting renal scarring. Indeed, the severity of a patient’s coronary artery lesion indicates the severity of childhood KD according to the current criteria [2, 3, 6, 7, 21]. The absence of a significant association between urinalysis data and renal involvement may be the result of transient changes in urine and the timing of urine collection.
Because the follow-up period in our study was limited to 6 months, we have no evidence of deterioration of renal function related to KD in these patients. Further research to investigate the utility of long-term monitoring of patients with renal scarring is warranted because hypertension, renal failure, or end-stage kidney disease are well-known sequelae of scarring [34, 35]. These patients should avoid renal toxicity factors that might cause rapidly progressive renal failure [36]. Strong evidence on patients with a history of KD who have reached adulthood [37] suggests that even those patients with KD and only mild coronary artery lesions should be followed up over the long term to screen for coronary artery disease or the development of premature atherosclerosis.
In conclusion, this is the first prospective, observational study to use DMSA renal SPECT to detect previously undiscovered renal inflammation in childhood KD. The high incidence of renal involvement and its subsequent scar formation warrant a large-scale follow-up study to verify the long-term clinical impact.
References
Kawasaki T (1967) Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children: Clinical Observation of 50 Cases (In Japanese). Jpn J Allergol 16:178–222
Brogan PA, Bose A, Burgner D, Shingadia D, Tulloh R, Michie C, Klein N, Booy R, Levin M, Dillon MJ (2002) Kawasaki disease: an evidence based approach to diagnosis, treatment, and proposals for future research. Arch Dis Child 86:286–290
Burns JC, Glode MP (2004) Kawasaki syndrome. Lancet 364:533–544
Amano S, Hazama F, Hamashima Y (1979) Pathology of Kawasaki disease: I. Pathology and morphogenesis of the vascular changes. Jpn Circ J 43:633–643
Amano S, Hazama F, Kubagawa H, Tasaka K, Haebara H, Hamashima Y (1980) General pathology of Kawasaki disease: On the morphological alterations corresponding to the clinical manifestations. Acta Pathol Jpn 30:681–694
Dajani AS, Taubert KA, Takahashi M, Bierman FZ, Freed MD, Ferrieri P, Gerber M, Shulman ST, Karchmer AW, Wilson W (1994) Guidelines for long-term management of patients with Kawasaki disease. Report from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 89:916–922
Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, Shulman ST, Bolger AF, Ferrieri P, Baltimore RS, Wilson WR, Baddour LM, Levison ME, Pallasch TJ, Falace DA, Taubert KA (2004) Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics 114:1708–1733
Melish ME, Hicks RM, Larson EJ (1976) Mucocutaneous lymph node syndrome in the United States. Am J Dis Child 130:599–607
Nardi PM, Haller JO, Friedman AP, Slovis TL, Schaffer RM (1985) Renal manifestations of Kawasaki’s disease. Pediatr Radiol 15:116–118
Ferriero DM, Wolfsdorf JI (1981) Hemolytic uremic syndrome associated with Kawasaki disease. Pediatrics 68:405–406
Foster BJ, Bernard C, Drummond KN (2000) Kawasaki disease complicated by renal artery stenosis. Arch Dis Child 83:253–255
Bonany PJ, Bilkis MD, Gallo G, Lago N, Dennehy MV, Sosa del Valle JM, Vallejo G, Canepa C (2002) Acute renal failure in typical Kawasaki disease. Pediatr Nephrol 17:329–331
Veiga PA, Pieroni D, Baier W, Feld LG (1992) Association of Kawasaki disease and interstitial nephritis. Pediatr Nephrol 6:421–423
Mac Ardle BM, Chambers TL, Weller SD, Tribe CR (1983) Acute renal failure in Kawasaki disease. J R Soc Med 76:615–616
Roberti I, Reisman L, Churg J (1993) Vasculitis in childhood. Pediatr Nephrol 7:479–489
Jakobsson B, Nolstedt L, Svensson L, Soderlundh S, Berg U (1992) 99mTechnetium-dimercaptosuccinic acid scan in the diagnosis of acute pyelonephritis in children: relation to clinical and radiological findings. Pediatr Nephrol 6:328–334
Majd M, Rushton HG, Chandra R, Andrich MP, Tardif CP, Rashti F (1996) Technetium-99m-DMSA renal cortical scintigraphy to detect experimental acute pyelonephritis in piglets: comparison of planar (pinhole) and SPECT imaging. J Nucl Med 37:1731–1734
Linne T, Fituri O, Escobar-Billing R, Karlsson A, Wikstad I, Aperia A, Tullus K (1994) Functional parameters and 99mtechnetium-dimercaptosuccinic acid scan in acute pyelonephritis. Pediatr Nephrol 8:694–699
Stokland E, Hellstrom M, Jakobsson B, Sixt R (1999) Imaging of renal scarring. Acta Paediatr Suppl 88:13–21
Council on Cardiovascular Disease in the Young, Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, American Heart Association (2001) Diagnostic guidelines for Kawasaki disease. Circulation 103:335–336
Dajani AS, Taubert KA, Gerber MA, Shulman ST, Ferrieri P, Freed M, Takahashi M, Bierman FZ, Karchmer AW, Wilson W (1993) Diagnosis and therapy of Kawasaki disease in children. Circulation 87:1776–1780
Nicholson JF, Pesce MA (2004) Reference ranges for laboratory tests and procedure. In Behrman RE, Kliegman RM, Jenson HB (eds) Nelson textbook of pediatrics, 17th edn, WB Saunders, Philadelphia, pp 2396–2426
Chiou YY, Wang ST, Tang MJ, Lee BF, Chiu NT (2001) Renal fibrosis: Prediction from acute pyelonephritis focus volume measured at 99mTc Dimercaptosuccinic acid SPECT. Radiology 221:366–370
Wang YT, Chiu NT, Chen MJ, Huang JJ, Chou HH, Chiou YY (2005) Correlation of renal ultrasonographic findings with inflammatory volume from dimercaptosuccinic acid renal scans in children with acute pyelonephritis. J Urol 173:190–194
Jakobsson B, Svensson L (1997) Transient pyelonephritic changes on 99mTechnetium-dimercaptosuccinic acid scan for at least five months after infection. Acta Paediatr 86:803–807
Report of the Subcommittee on Standardization of Diagnostic Criteria and Reporting of Coronary Artery Lesions in Kawasaki Disease (1984) Tokyo, Japan: Research Committee on Kawasaki disease, Ministry of Health and Welfare
Salcedo JR, Greenberg L, Kapur S (1988) Renal histology of mucocutaneous lymph node syndrome (Kawasaki disease). Clin Nephrol 29:47–51
Ogawa H (1985) Kidney pathology in muco-cutaneous lymphnode syndrome. Jpn J Nephrol 27:1229–1237
Rowley AH, Shulman ST, Mask CA, Finn LS, Terai M, Baker SC (2000) IgA plasma cell infiltration of proximal respiratory tract, pancreas, kidney, and coronary artery in acute Kawasaki disease. J Infect Dis 182:1183–1191
Grunebaum E, Blank M, Cohen S, Afek A, Kopolovic J, Meroni PL, Youinou P, Shoenfeld Y (2002) The role of anti-endothelial cell antibodies in Kawasaki disease-in vitro and in vivo studies. Clin Exp Immunol 130:233–240
Watanabe T, Abe Y, Sato S, Uehara Y, IKeno K, Abe T (2006) Hyponatremia in Kawasaki disease. Pediatr Nephrol 21:778–781
Ohta K, Seno A, Shintani N, Kato E, Yachie A, Seki H, Miyawaki T, Taniguchi N (1993) Increased levels of urinary interleukin-6 in Kawasaki disease. Eur J Pediatr 152:647–649
Jibiki T, Terai M, Kohno Y (2004) High concentrations of interleukin-8 and monocyte chemoattractant protein-1 in urine of patients with acute Kawasaki disease. Eur J Pediatr 163:749–750
Hellerstein S (2000) Long-term consequences of urinary tract infections. Curr Opin Pediatr 12:125–128
Rushton HG (1997) Urinary tract infections in children. Epidemiology, evaluation, and management. Pediatr Clin North Am 44:1133–1169
Resch M, Banas B, Endemann D, Mack M, Riegger GAJ, Gröne HJ, Kramer BK (2006) Exanthema and acute anuric renal failure. Clin Nephrol 65:361–363
Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, Kazue T, Eto G, Yamakawa R (1996) Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation 94:1379–1385
Conflict of interest statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Financial support: Grants from the National Cheng Kung University Medical Center.
Rights and permissions
About this article
Cite this article
Wang, JN., Chiou, YY., Chiu, NT. et al. Renal scarring sequelae in childhood Kawasaki disease. Pediatr Nephrol 22, 684–689 (2007). https://doi.org/10.1007/s00467-006-0385-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-006-0385-y