Abstract
The impact of chronological age on longitudinal body growth from early childhood through adolescence using detailed anthropometric methods has not yet been studied in children with chronic kidney disease (CKD). We have evaluated growth failure by measuring four components of linear growth: body height (HT), sitting height (SHT), arm length (AL) and leg length (LL). Data were prospectively collected for up to 7 years on 190 boys (3–21 years old) with congenital or hereditary CKD (all had developed at least stage 2 CKD by the age of 10 years). Patients showed the most severe growth failure in early childhood, followed by an acceleration in growth in pre-puberty, a slowing-down of growth at puberty, as expected, and thereafter a late speeding-up of growth until early adulthood. This pattern was observed irrespective of the degree of CKD and different treatment modalities, such as conservative treatment, recombinant human growth hormone (rhGH) therapy or transplantation. LL showed the most dynamic growth changes of all the parameters evaluated and emerged as the best indicator of statural growth in children with CKD. A specific age-dependent pattern of physical growth was identified in pediatric male CKD patients. This growth pattern should be considered in the evaluation of individual growth and the assessment of treatment efficacy such as rhGH therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Growth failure in pediatric patients with chronic kidney disease (CKD) is a major obstacle for full rehabilitation and may result in severely diminished adult height. Healthy children typically exhibit a maximal growth velocity in infancy, followed by a period of continuous slower growth and thereafter a growth spurt during puberty. The disturbance in the growth pattern observed in pediatric patients with CKD is due to a variety of factors, such as malnutrition, anemia, metabolic acidosis and hormonal disorders [1]. The age at onset of kidney disease plays a major role, and the loss of relative height is greatest in the first year of life [2, 3]. Puberty is frequently delayed in adolescents with CKD, and the total pubertal height gain is reduced. The type of underlying renal disease, renal function and gender differences are further important determinants.
Current strategies aimed at optimizing growth in patients with CKD include the correction of metabolic disturbances, renal replacement therapy in the form of dialysis and transplantation, nutritional supplementation with tube feeding or gastrostomy and treatment with recombinant human growth hormone (rhGH). Previous studies have shown that children do not grow well on dialysis [4] and may experience catch-up growth following transplantation [5] and rhGH treatment [6]. However, too little is known about the evolution of growth failure from early childhood through adolescence in pediatric patients with CKD. Previous studies were hampered by small numbers of patients, non-homogeneous patient groups, limited follow-up time and a lack of evaluation of segmental growth (trunk, arm and leg length). Measurements of body height alone may not adequately reflect the true influence of CKD and treatment modalities on growth, since total body height is a composite variable, and different body parts have their own timing and intensity of growth. The trunk and the legs develop in all growth phases quite differently [7], and variable height is thus composed of two heterogeneous growth components [8]. The evaluation of segmental growth is of special interest as body disproportion has been demonstrated as an important factor in cardiovascular disease in the general population [9, 10].
We have therefore conducted a prospective, detailed anthropometric study in a large cohort of pediatric patients from two centers with a similar approach to treatment. In an effort to investigate a homogenous group, we limited the study to boys with congenital and hereditary CKD leading to renal failure before the age of 10 years.
Patients and methods
Patients
The institutional review boards approved the study. Informed patient’s and/or caregiver’s consent was obtained prior to enrollment. From May 1998 to October 2005, a total of 190 male patients (age range: 3–21 years) with congenital or hereditary CKD stage 2 and higher [11] [glomerular filtration rate (GFR) < 90 ml/min per 1.73 m2] underwent prospective anthropometric measurements. Patients were followed up in two pediatric nephrology centers (Charité University Hospital, Berlin and Hannover Medical School). GFR was determined by the Schwartz formula [12]. Patients with height-affecting skeletal abnormalities, such as scoliosis, were excluded. Underlying renal diseases were: obstructive uropathy with/without vesicoureteral reflux (VUR; 36%), renal dysplasia/hypoplasia (28%), nephronophthisis (9%), congenital nephrotic syndrome (7%), hereditary glomerulopathies (6%), cystinosis (5%), polycystic kidney disease (5%) and others (4%).
At initial anthropometric measurement, 80/190 (42%) of the patients were on conservative treatment; 24/190 (13%) patients were on dialysis, starting dialysis at a median age of 7.6 years (range: 0.2–16.82 years); 86/190 (45%) patients had been transplanted at least once (median age at first transplant: 8.8 years; range: 2.05–14.55 years) and had from one to three functioning renal allografts with a median GFR of 49 ml/min per 1.73 m2.
Seventy-four (39%) of the 190 children had been treated with rhGH. The median age at the start of rhGH therapy was 7.5 years (semi-interquartile range: 3.3 years). The median duration of rhGH therapy was 2.6 years (semi-interquartile range: 1.2 years).
During repeated anthropometric measurements, 26% of 148 patients progressed through different stages of CKD, resulting in alterations in the treatment, such as conservative management of CKD, dialysis and transplantation. The greatest proportion of treatment changes occurred in those patients on dialysis (71% of 17 patients with repeated measurements).
The median age of all 190 patients at the initial measurement was 10.5 years (range: 3.02–19.4 years), and the median GFR at initiation of the study was 37 ml/min per 1.73 m2. Altogether, 614 yearly anthropometric measurements were performed. Nine age groups were divided into 2-year intervals beginning with 3–4, 5–6, continuing up to 18+ years.
Anthropometric measurements of longitudinal body dimensions included:
-
Total body height (HT) – stature was measured as the maximal distance between the vertex of the head, which was held in the Frankfurt plane [13], and the floor. The patients were measured barefoot, standing erect with their heels together and arms hanging naturally by their sides.
-
Sitting height (SHT) was measured as the distance between the seat surface and the vertex, with the head in the Frankfurt plane. The patients were seated as straight as possible with their back against the wall.
-
Arm length (AL) was measured as the distance between the acromion process and the tip of the third finger of the left hand.
-
Leg length (LL) was measured in the same position as stature, as the distance between the spina iliaca anterior superior on the left-hand side and the floor.
The same investigator (M.Ž) took all measurements, as recommended by the International Biological Program [13] with standardized equipment (Dr. Keller I Stadiometer-Limbach-Oberfrohna, Germany; Siber Hegner Anthropometer-Zürich, Switzerland). The accuracy of the measurements was within 1 mm. Z-scores (standard deviation scores, SDS) were calculated with reference limits derived from 5155 healthy children aged 3–18 years [14].
In order to analyze the effect of the degree of renal failure on the growth pattern, we divided the patients into two groups according to their median GFR. The mean GFR during the observation period of the whole study cohort of 43 ml/min per 1.73 m2 was arbitrarily chosen as a cut-off. The median GFR in the group with “good” and “poor” renal function was 59 (semi-interquartile range: 10.6) and 30 (semi-interquartile range: 13.1) ml/min per 1.73 m2, respectively.
Statistics
SDS values for the observed parameters were calculated according to the equation
where xi is the individual value of the patient, and xs and SD are the mean and standard deviation values for age and sex-matched healthy peers, respectively.
To describe the group characteristics, we used the median (M) as the measure of central tendency and the semi-interquartile range (Q) as the measure of variability
where Q is one-half the distance between the first and third quartile points.
The normality of the distribution was evaluated by the Kolmogornov-Smirnov test in each age cohort for each parameter. As the distribution did not differ significantly from normal, parametric methods for analysis were applied. Means of variables were compared in each age cohort with the paired sample t-test, if the variance of groups was equal (Levan test of homogenicity of variance). In cases where the variance of the groups was not equal, the Welch and Brown-Forsythe test was applied. The correlation coefficients in each age cohort and for each pair of variables were analyzed with the paired samples t-test.
Age-dependent changes were determined with an analysis of variance post-hoc least square.SPSS ver. 13 software (SPSS, Chicago, Ill.) was used for all statistical calculations and for the generation of growth curves with the spline function.
Results
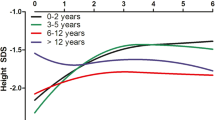
Growth deficit and disproportion was more pronounced in young children (3–6 years) than in school-aged children (7–12 years) (Fig. 1). Children in early puberty showed a continuous decrease in all four longitudinal dimensions, followed thereafter by an increase until late adolescence (Fig. 1, Table 1).
Total body height (HT) was most severely impaired during early childhood with a mean HT-SDS of −2.2 (Table 1, Fig. 1). In the age cohort 3–4 years, HT-SDS in 59% of the patients was below −2. During pre-puberty, HT-SDS increased significantly (p < 0.01) from −2.2 to −1.6, reaching a maximum in the age cohort 11–12 years, with only 32% of patients having a HT-SDS below −2. The expected pubertal period was characterized by a delay in statural growth, leading to a slowing-down when compared to healthy peers (p < 0.05). Mean HT-SDS decreased from the age group 11–12 years to 15–16 years from −1.6 to −2.2 (Tables 1, 2, Fig. 1). In the age cohort 15–16 years, 46% had a HT-SDS below −2. Finally, SDS increased in late puberty (patients 17–18 years) to −1.9 in; HT-SDS below −2 was only found in 32% of these patients.
Sitting height (SH) was the best preserved parameter of all longitudinal dimensions. SH-SDS reached the lowest mean value of −1.6 in age cohorts 7–8 and 15–16 years for which 32 and 37% were below −2 SDS (Table 1, Fig. 1). The highest SH-SDS was documented in the 11- to 12-year-old cohort (−1.1 SDS), with only 19% of patients below −2 SDS. Significant differences in SH-SDS were found between the peaks in the age cohorts 11–14 years and the troughs in the age cohorts 7–8 and 15–16 years (Fig. 1, Table 2).
Arm length was the most stable of all observed parameters (range: −2.1 to −1.6 SDS) (Fig. 1, Tables 1, 2). The AL-SDS curve pattern was similar to that of stature. An acceleration in growth occurred in pre-puberty when AL-SDS increased significantly from childhood until the late pre-pubertal age (11–12 years) from −2.1 to −1.6 (p < 0.05) (Table 2). This was followed by a slowing-down period: AL-SDS decreased significantly (p < 0.05) to −2 until the age of 15–16 years. The late pubertal increase was not significant in AL-SDS, reaching −1.8 in the 17- to 18-year-old cohort and −1.6 SDS at adult ages. The percentages of cases below −2 SDS ranged from 28% in subjects >18 years to 47% in the age cohort of 3–4 years.
LL showed the most pronounced age-dependent changes of all four parameters (Fig. 1, Table 2). In the age cohort of 5–6 years, mean LL-SDS was −2.7; in the age cohort of 17–18 years, −1.4; in the age cohort >18 years, only −1.5 SDS. The percentage of patients below −2 SDS decreased from 63 to 11% from early childhood to adulthood. The most marked acceleration in growth was observed in the pre-pubertal age groups 5–6 up to 11–12 years (p < 0.005), where LL-SDS increased by 0.8 SDS (Fig. 1, Table 1). During the expected onset of puberty of healthy children (11–14 years) the mean LL-SDS decreased slightly from −1.8 to −2 SDS. After this phase of decreased leg growth, another speeding-up period was observed, although it was not as pronounced as in pre-puberty. However, growth lasted longer than in healthy peers, with a consecutive gain in LL from the age of 16 years to adulthood.
A comparison of mean SDS values for paired parameters of the longitudinal dimension in each age cohort (Table 1) confirmed that differences were greatest in childhood and smallest in the oldest age cohorts. The most dramatic differences were seen between HT- and SHT-SDS, followed by differences between extremity SDS (arm and legs). HT and AL showed the greatest similarities; differences were significant only in the pre-school age (Table 1).
The correlation between HT and the other three observed parameters showed an age dependence (Fig. 2). While the correlation between extremities and HT was high, with a relatively slight oscillation in different age cohorts, the correlation between HT and STH varied between moderate (r = 0.75) in early childhood and high (r = 0.92) in the early pubertal years (Fig. 2). The correlation was highest between HT-and LL-SDS and smallest between SHT-and LL-SDS.
The coefficient of determination (R2) was higher between stature (HT) and LL (0.85) than between HT and AL (0.82) or SHT (0.77); LL emerged as the best indicator for statural growth in children with CKD.
HT-SDS values were significantly higher in patients with good kidney function than in those with poor kidney function (p < 0.001) (Fig. 3a). However, the same pattern of SDS curves was observed for both groups: early acceleration in growth in pre-puberty, slowing-down at early pubertal age and a late acceleration in growth at adolescence. Interestingly, the mean annual pre-pubertal growth gain (age: 5–10 years) was almost identical in patients, regardless of their kidney function (56 mm for HT, 25 mm for SHT, 26 mm for AL and 37 mm for LL in the group with poor renal function compared to a growth gain of 60 mm for HT, 26 mm for SHT, 27 mm for AL and 40 mm for LL in the group with good renal function).
Mean standard deviation scores (SDS) for stature by age cohorts in boys with congenital and hereditary chronic kidney disease (CKD): a according to the kidney function [glomerular filtration rate (GFR) <43 ml/min per 1.73 m2 vs. GFR >43 ml/min per 1.73 m2], b according to conservative treatment vs. renal transplantation
A comparison of the growth pattern in patients with conservative treatment and transplanted patients showed the same basic shape as already described (Fig. 3b).
The effect of rhGH therapy on HT in all patients with a follow-up of more than 1 year (n = 25) is presented in Fig. 4a,b. Not surprisingly, the stature of patients with rhGH therapy was more severely impaired than that of patients without rhGH therapy. Interestingly, both groups of patients showed a similar pattern of growth retardation. Nonetheless, the degree at which growth was speeded up in pre-puberty and late adolescence was more pronounced in patients treated with rhGH than in those who were not; the average annual HT increase was higher (p < 0.001) in patients during rhGH treatment (60 mm) than in patients never receiving rhGH (47 mm).
Mean total body height standard deviation scores (SDS) (a) and mean annual change (mm) in total body height (b) in boys with congenital and hereditary chronic kidney disease (CKD) during recombinant human growth hormone (rhGH) therapy and in boys with congenital and hereditary CKD who never received rhGH
Growth velocity during different the time intervals before and after renal transplantation (RTx) is shown in Fig. 5. The phase of “end stage” CKD 1 year before renal transplantation was associated with the lowest average growth velocity (GV) of 48 mm/year. In the year of transplantation and the years thereafter, GV was increasing, and in the first and second year after RTx mean GV was 55 mm. The small sample size (25 patients with appropriate data before and after RTx) and the wide age span of 3–19 years were limiting factors for statistically detailed analyses. In the pre-pubertal age, mean GV was 73 mm for patients in the first and second year after RTx (nine cases) and only 52 mm in the year before and the year of RTx (five cases). In the age span 11–16 years, differences in GV between pre- and post-RTx were more marked (the year before RTx, the mean GV was 41 mm vs. 67 mm in the group in the second year after RTx).
Discussion
In this prospective anthropometric study of 190 male pediatric patients with congenital and hereditary CKD leading to renal failure before the age of 10 years, a distinct age-related pattern of impaired longitudinal growth could be identified. Three novel observations were made.
Firstly, four periods of growth impairment were identified. Boys under 6 years of age showed the most severe growth deficit in HT, AL and LL (early growth deficit). From the age of 5 to 12 years, patients experienced a consecutive and significant increase in HT and extremity length (early speeding-up of growth). During the time of the expected onset of puberty in healthy children, patients aged 13–16 years showed a significant decrease in the SDS of all measured anthropometric parameters (slowing-down of growth). Thereafter, from 16 years to adulthood, these male patients again had an increase in anthropometric SDS values (late speeding-up of growth) when compared to healthy children.
Previous publications have described aspects of this growth pattern, either focusing on infancy [15, 16], pre-puberty [2, 17–24] or puberty [25]. Unfortunately, these studies were hampered by relatively small numbers of patients and non-homogeneous patient groups (inclusion of male and female patients, congenital and acquired CKD and different onset of the disease). Furthermore, a wide age range stratification was usually used (e.g. all children under 7 years [18, 24, 26]. There is general consensus that infancy and early childhood represent the most vulnerable growth periods in children with CKD [3, 15, 22, 24, 27–29]. Ismaili et al. [28] showed that mean HT-SDS decreased from −0.7 in newborns to −2.4 at the age of 2 years in infants with CKD caused by renal dysplasia. Schaefer et al. [30] analyzed the growth of 321 prepuberal patients with CRF: mean height for newborns was 49.8 cm and 79 cm for 2-year-old children, which corresponded to −0.039 and −2.67 SDS, respectively, using the references of Prader et al. [31]. In our study we documented data at birth from 108/190 patients. Interestingly, the data are very similar to the above-mentioned data of Ismaili et al. and Schaefer et al. [28, 30]: mean birth length was 49.5 ± 4.2 cm. We conclude that the main reason for the growth deficit in early childhood is due to growth failure in the infantile period.
No previous studies have investigated the evolution of growth failure over the whole pediatric age range from childhood to adulthood. The present study shows that growth kinetics differed during early and late puberty, beginning with a slowing-down of growth during early puberty. This delay of the onset of puberty is common in children with CKD [5, 32]. Rees et al. [17] noticed a delay in sexual maturity and a decline in HT-SDS at the time of the expected pubertal growth spurt in children who had had renal transplantation, with clear evidence of a beginning growth recovery in late adolescence. André et al. [25] noted that delayed pubertal growth in children with CKD was reflected by a decrease in HT-SDS at the age of 16 years (−2.4 ± 1.4 SDS). These findings correspond to our results in showing a statural slowing-down in the age span from 12 to 16 years, followed by a speeding-up of growth that continued until adulthood. The late pubertal acceleration in growth is explained by the fact that delayed puberty in boys with CKD is followed by a prolonged period of growth, occasionally even after 20 years of age [25, 33–35].
Secondly, the degree of kidney failure and treatment modalities, such as transplantation and rhGH treatment, did significantly influence the severity of growth impairment, but they did not alter the age dependent growth pattern (Figs. 3, 4 and 5).
Thus, a speeding-up of growth in pre-puberty in children with CKD cannot be attributed solely to an improvement in medical care. Similar changes occur in healthy children living in poor environments in developing countries: Adair [36] also observed the most severe growth retardation during the first two years of life and a speeding-up of growth at the time of the expected puberty . Pre-puberty seems to allow catch-up growth in both healthy and sick children. Rees et al. [17] described pre-pubertal speeding-up growth in children after renal transplantation, independent of medical treatment (high-dose steroids and azathioprine or low-dose steroids and cyclosporin). Conversely, Ellis [26] interpreted this phenomenon to a discontinuation or withdrawal of steroids within 1 year of transplantation in children receiving tacrolimus.
Thirdly, poor leg growth appears to be the most important factor for growth failure in pre-school aged boys with CKD. In patients aged 3–4 years, mean SHT-SDS was −1.4, while mean LL-SDS was −2.3, thus confirming the observation of parents that these children need a considerably bigger size of shirts than of trousers. In adulthood, SHT-SDS was −1.3 and LL-SDS −1.4. LL showed the most dynamic changes in children and adolescents with CKD. The correlation was highest between HT and LL and weakest between SH and LL, affirming previous auxological findings that the growth phases of trunk and legs are different [7]. This suggests that leg growth should be taken as the most sensitive indicator of linear growth in children with congenital CKD.
Anthropological studies have also confirmed the concept that impaired leg growth is a better indicator than HT or SHT for disturbed early childhood environmental circumstances such as malnutrition or chronic diseases [37–44]. An association between impaired leg growth and increased incidence of cardiovascular disease has been demonstrated in the general population [9, 10]. Since cardiovascular mortality is higher in children and adults with CKD compared to the healthy population, future studies will have to evaluate whether impaired leg growth is an indicator for a high cardiovascular risk in patients with CKD.
In this study we did not separate patients according to treatment modalities (conservative, dialysis and transplantation) as these change at a rapid pace in pediatric CKD. For example, the average duration of the first dialysis period in our patients was 1.23 ± 1.33 years, and 71% of the children on dialysis at the beginning of the study were transplanted at least once during the observation time.
In summary, comprehensive data of body measurements were prospectively collected (up to 7 years) in a large group of pediatric patients (190 boys) with congenital and hereditary CKD. With more than 600 yearly measurements in this cohort, we were able to analyze age-dependent growth during childhood and adolescence for the first time. Growth impairment in boys with congenital or hereditary CKD was age-dependent, with the most vulnerable period of longitudinal growth being early childhood. The catch-up potential was highest in pre-puberty. A slowing-down of growth was experienced, as expected, during early puberty, followed by a second speeding-up during late puberty.
Trunk growth had little synchronicity with leg growth. The catch-up potential was mainly determined by leg growth.
We conclude that growth impairment in boys with CKD follows an age-dependent pattern of distinct changes in segmental growth throughout childhood and adolescence. A good understanding of this pattern is of clinical relevance for the interpretation of growth curves and decisions on treatment options such as rhGH therapy.
References
Ledermann SE, Shaw V, Trompeter RS (1999) Long-term enteral nutrition in infants and young children with chronic renal failure. Pediatr Nephrol 13:870–875
Abitbol CL, Warady BA, Massie MD, Baluarte HJ, Fleischman LE, Geary DF, Kaiser BA, McEnery PT, Chan JC (1990) Linear growth and anthropometric and nutritional measurements in children with mild to moderate renal insufficiency: a report of the Growth Failure in Children with Renal Diseases Study. J Pediatr 116:S46–S54
Fivush BA, Jabs K, Neu AM, Sullivan EK, Feld L, Kohaut E, Fine R (1998) Chronic renal insufficiency in children and adolescents: the 1996 annual report of NAPRTCS. North American Pediatric Renal Transplant Cooperative Study. Pediatr Nephrol 12:328–337
Lerner GR, Warady BA, Sullivan EK, Alexander SR (1999) Chronic dialysis in children and adolescents. The 1996 annual report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Nephrol 13:404–417
Nissel R, Brazda I, Feneberg R, Wigger M, Greiner C, Querfeld U, Haffner D (2004) Effect of renal transplantation in childhood on longitudinal growth and adult height. Kidney Int 66:792–800
Haffner D, Schaefer F, Nissel R, Wuhl E, Tonshoff B, Mehls O (2000) Effect of growth hormone treatment on the adult height of children with chronic renal failure. German Study Group for Growth Hormone Treatment in Chronic Renal Failure. N Engl J Med 343:923–930
Sheehy A, Gasser T, Molinari L, Largo RH (1999) An analysis of variance of the pubertal and midgrowth spurts for length and width. Ann Hum Biol 26:309–331
Sheehy A, Gasser T, Molinari L, Largo RH (2000) Contribution of growth phases to adult size. Ann Hum Biol 27:281–298
Smith GD, Greenwood R, Gunnell D, Sweetnam P, Yarnell J, Elwood P (2001) Leg length, insulin resistance, and coronary heart disease risk: the Caerphilly Study. J Epidemiol Community Health 55:867–872
Gunnell D, Whitley E, Upton MN, McConnachie A, Smith GD, Watt GC (2003) Associations of height, leg length, and lung function with cardiovascular risk factors in the Midspan Family Study. J Epidemiol Community Health 57:141–146
Hogg RJ, Furth S, Lemley KV, Portman R, Schwartz GJ, Coresh J, Balk E, Lau J, Levin A, Kausz AT, Eknoyan G, Levey AS (2003) National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative clinical practice guidelines for chronic kidney disease in children and adolescents: evaluation, classification, and stratification. Pediatrics 111:1416–1421
Schwartz GJ, Brion LP, Spitzer A (1987) The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 34:571–590
Weiner JS, Lourie JA (1981) Practical human biology. Academic Press, London
Živičnjak M, Narancic NS, Szirovicza L, Franke D, Hrenovic J, Bisof V (2003) Gender-specific growth patterns for stature, sitting height and limbs length in Croatian children and youth (3 to 18 years of age). Coll Antropol 27:321–334
Abitbol CL, Zilleruelo G, Montane B, Strauss J (1993) Growth of uremic infants on forced feeding regimens. Pediatr Nephrol 7:173–177
Karlberg J, Schaefer F, Hennicke M, Wingen AM, Rigden S, Mehls O (1996) Early age-dependent growth impairment in chronic renal failure. European Study Group for Nutritional Treatment of Chronic Renal Failure in Childhood. Pediatr Nephrol 10:283–287
Rees L, Greene SA, Adlard P, Jones J, Haycock GB, Rigden SP, Preece M, Chantler C (1988) Growth and endocrine function after renal transplantation. Arch Dis Child 63:1326–1332
Fennell RS, Moles M, Iravani A, Walker RD, Pfaff W, Howard RJ, Capen RC, Carter RL, Richard GA (1990) Growth in children following kidney transplantation. Pediatr Nephrol 4:335–339
Tejani A, Fine R, Alexander S, Harmon W, Stablein D (1993) Factors predictive of sustained growth in children after renal transplantation. The North American Pediatric Renal Transplant Cooperative Study. J Pediatr 122:397–402
Fine RN (1997) Growth post renal-transplantation in children: lessons from the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). Pediatr Transplant 1:85–89
Tyden G, Berg U, Bohlin AB, Sandberg J (1997) Renal transplantation in children less than two years old. Transplantation 63:554–558
Van Dyck M, Bilem N, Proesmans W (1999) Conservative treatment for chronic renal failure from birth: a 3-year follow-up study. Pediatr Nephrol 13:865–869
Kari JA, Gonzalez C, Ledermann SE, Shaw V, Rees L (2000) Outcome and growth of infants with severe chronic renal failure. Kidney Int 57:1681–1687
Englund MS, Tyden G, Wikstad I, Berg UB (2003) Growth impairment at renal transplantation-a determinant of growth and final height. Pediatr Transplant 7:192–199
Andre JL, Bourquard R, Guillemin F, Krier MJ, Briancon S (2003) Final height in children with chronic renal failure who have not received growth hormone. Pediatr Nephrol 18:685–691
Ellis D (2000) Growth and renal function after steroid-free tacrolimus-based immunosuppression in children with renal transplants. Pediatr Nephrol 14:689–694
Betts PR, Magrath G (1974) Growth pattern and dietary intake of children with chronic renal insufficiency. Br Med J 2:189–193
Ismaili K, Schurmans T, Wissing KM, Hall M, Van Aelst C, Janssen F (2001) Early prognostic factors of infants with chronic renal failure caused by renal dysplasia. Pediatr Nephrol 16:260–264
Furth SL, Stablein D, Fine RN, Powe NR, Fivush BA (2002) Adverse clinical outcomes associated with short stature at dialysis initiation: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatrics 109:909–913
Schaefer F, Wingen AM, Hennicke M, Rigden S, Mehls O (1996) Growth charts for prepubertal children with chronic renal failure due to congenital renal disorders. European Study Group for Nutritional Treatment of Chronic Renal Failure in Childhood. Pediatr Nephrol 10:288–293
Prader A, Largo RH, Molinari L, Issler C (1989) Physical growth of Swiss children from birth to 20 years of age. First Zurich longitudinal study of growth and development. Helv Paediatr Acta Suppl 52:1–125
Schaefer F, Seidel C, Binding A, Gasser T, Largo RH, Prader A, Scharer K (1990) Pubertal growth in chronic renal failure. Pediatr Res 28:5–10
Rizzoni G, Broyer M, Guest G, Fine R, Holliday MA (1986) Growth retardation in children with chronic renal disease: scope of the problem. Am J Kidney Dis 7:256–261
Diemen-Steenvoorde R, Donckerwolcke RA, Brackel H, Wolff ED, de Jong MC (1987) Growth and sexual maturation in children after kidney transplantation. J Pediatr 110:351–356
Diemen-Steenvoorde R, Donckerwolcke RA (1988) Growth and sexual maturation in paediatric patients treated by dialysis and following kidney transplantation. Acta Paediatr Scand Suppl 343:109–177
Adair LS (1999) Filipino children exhibit catch-Up growth from age 2 to 12 years. J Nutr 129:1140–1148
Jantz LM, Jantz RL (1999) Secular change in long bone length and proportion in the United States, 1800–1970. Am J Phys Anthropol 110:57–67
Zhang X, Huang Z (1988) The second national growth and development survey of children in China, 1985: children 0 to 7 years. Ann Hum Biol 15:289–305
Bolzan AG, Guimarey LM, Pucciarelli HM (1993) Growth and sex dimorphism in school children according to their father’s occupation. Arch Latinoam Nutr 43:132–138
Ali MA, Uetake T, Ohtsuki F (2000) Secular changes in relative leg length in post-war Japan. Am J Human Biol 12:405–416
Leitch I (2001) Growth and health. 1951. Int J Epidemiol 30:212–216
Bogin B, Smith P, Orden AB, Varela Silva MI, Loucky J (2002) Rapid change in height and body proportions of Maya American children. Am J Hum Biol 14:753–761
Tanner JM, Hayashi T, Preece MA, Cameron N (1982) Increase in length of leg relative to trunk in Japanese children and adults from 1957 to 1977: comparison with British and with Japanese Americans. Ann Hum Biol 9:411–423
Lawlor DA, Ebrahim S, Davey SG (2002) The association between components of adult height and Type II diabetes and insulin resistance: British Women’s Heart and Health Study. Diabetologia 45:1097–1106
Acknowledgements
We thank all patients and their families for their cooperation. We are indebted to the doctors and nurses in Hannover and Berlin for their help and to Darlene Poulin for her valuable editing of the English language. This study was supported by an institutional grant from Pfizer, Karlsruhe, Germany and clinical research funds of the Charité University Hospital Berlin.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Živičnjak, M., Franke, D., Filler, G. et al. Growth impairment shows an age-dependent pattern in boys with chronic kidney disease. Pediatr Nephrol 22, 420–429 (2007). https://doi.org/10.1007/s00467-006-0345-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-006-0345-6