Abstract
Nephrocalcinosis (NC) occurs frequently in preterm neonates. A high U-calcium/citrate is one of the contributing factors to the development of NC. In stone-forming children and adults citrate supplementation is a successful preventive therapy. In this randomized controlled trial the effect of citrate therapy was studied on the development of NC in preterm neonates with a gestational age <32 weeks. Thirty-eight preterm neonates (mean gestational age 29.8 weeks (SD 1.6), mean birth weight 1,300 g (SD 351) were treated with sodium citrate (0.52 mmol/kg/day in four doses) from day 8 of life until at term and 36 preterm neonates (mean gestational age 29.6 weeks (SD 1.6), mean birth weight 1,282 g (SD 256) were not treated. U-calcium, U-creatinine, U-citrate and U-pH were measured at day 7, 14, 21, 28 of life and at term. Renal ultrasonography (US) was performed at term. U-citrate/creatinine and U-pH were significantly higher and U-calcium/citrate was significantly lower in the citrate group at day 14, 21 and 28 compared with the control group (P<0.05). Complications of citrate administration were not encountered, however the incidence of NC was not significantly different in the treated (34%) compared with the control group (44%), P=0.37. Preterm neonates treated with citrate in the first months of life have higher U-citrate/creatinine and lower U-calcium/citrate compared with controls. Sodium citrate therapy in a dosage of 0.52 mmol/kg/day is safe but does not prevent NC. Whether a higher dose or potassium citrate decreases the incidence of NC should be evaluated in further studies.
Similar content being viewed by others


Avoid common mistakes on your manuscript.
Introduction
Nephrocalcinosis (NC) occurs frequently in preterm neonates. The incidence varies between 17 and 64%, depending on the population studied, age at examination and ultrasonographic (US) criteria [1–8]. Although in most patients NC disappears spontaneously in months to years, some patients suffer from nephrolithiasis with obstruction of the urinary tract [3, 9]. Besides, later in life high blood pressure and impaired glomerular and distal tubular function may occur more frequently than in healthy children [10–12].
NC is a result of spontaneous and therapy induced imbalance between promoters (e.g., calcium, oxalate, uric acid) and inhibitors (e.g., citrate, magnesium) of crystallization in urine [13, 14]. Citrate inhibits the crystallization process of calcium-oxalate crystals [15–19]. Besides, citrate forms a more soluble complex with calcium and thereby reduces the formation of less soluble calcium-oxalate or calcium-phosphate complexes.
Several studies demonstrate hypercalciuria, hypocitraturia, hyperoxaluria and hyperuricosuria in preterm neonates with NC [13, 20–22]. Various factors contribute to these urinary findings, including immaturity of renal function, metabolic acidosis, dietary intake and presence and treatment of chronic lung disease with diuretic therapy, such as furosemide, and corticosteroids [1, 13, 23].
In older children and adults with hypercalciuria, hypocitraturia, hyperoxaluria or hyperuricosuria citrate administration is an established therapy to prevent stone formation [15–18, 24].
This is the first trial to study the effect of citrate administration on the development of NC in preterm neonates. In a randomized controlled study we examined the possibility of preventing NC in preterm neonates by enteral administration of sodium citrate.
Methods
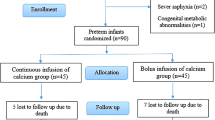
The study was performed in the neonatal care unit of Leiden University Medical Centre and Juliana Children’s Hospital, The Hague. Preterm neonates with a gestational age <32 weeks admitted to these hospitals in the first week of life were eligible for the study. Patients from outside the usual referral region of these hospitals were excluded from the study, as were those who were transferred to another hospital during the study period or died before 7 days of age. Informed consent was obtained after oral and written information had been given. The study protocol was approved by the ethics committees of the two participating hospitals.
Patients were randomly assigned to receive sodium citrate or no medication. Citrate was administered as trisodium citrate (molecule weight of 294 g/mol; osmolarity of 270 osmol/l; 0.11 mmol/ml) in a total dose of 0.52 mmol/kg/day given in four divided doses of 0.13 mmol/kg/dose from day 8 after birth until at term (post-conceptional age of 38–42 weeks), at which time a renal US was performed. Citrate was not administered on days when enteral feeding was less than 24 ml/kg/day or on days when the base excess was more than 5 accompanied by a pH higher than 7.45 when no other explanation than citrate administration for this could be found.
The following clinical data were recorded: gestational age, birth weight, days of treatment with furosemide, thiazide, theophylline, caffeine or corticosteroids (which was used in patients with apparent respiratory problems), days with diarrhea, retention of feeding, vomiting and presence of chronic lung disease (CLD). CLD was defined as need for oxygen and corresponding abnormalities on the X-thorax at the age of 36 weeks post-conception.
At day 7, 14, 21 and 28 of life and at term, urine samples were collected to measure calcium, creatinine, citrate, phosphate, magnesium, oxalate and pH (performed directly after the neonate had urinated). In addition, blood gas was performed. At day 7 and 28 of life and at term, calcium and creatinine levels were assessed in serum.
Urine aliquots were collected in attached plastic bags. The pH was measured by dipstick method immediately after urine was collected. Calcium, phosphate, creatinine and citrate were measured by colorimetric method (Hitachi analyser Roche diagnostics, Almere, The Netherlands). Oxalate was measured manually by a coloric photometric method (Instruchemie Hilversum, The Netherlands). Magnesium was determined by atomic absorption spectrophotometry. Blood gas values were measured using electrodes (Chiaron Diagnostics 865, Mijdrecht, The Netherlands).
At term US of the kidneys was performed to detect NC. Two pediatric radiologists, one in each centre, performed the US examination with Toshiba SSA-140A equipment in Leiden and a HTL 5000 scanner (ATL HDI 5000; Bethel, Wash.) in The Hague, using a 7-to 7.5-MHz small-part transducer. The radiologists were unaware of the clinical history and treatment of the patients. Transverse and longitudinal images were made of both kidneys. NC was defined as the presence of bright reflections in the medulla or cortex that were reproducible both in transverse and longitudinal directions with or without acoustic shadowing.
Using the data from our observational study performed, with an incidence of NC of 40%, a power analysis was performed [5]. This analysis showed that 72 patients would be needed to demonstrate a difference of 30% (40% versus 10% NC) with an alpha of 5% and a power of 80%. The randomization lists were made by the statistic department, which was blinded for the ascription of the groups. Data were analyzed using SPSS statistical software. Groups were compared using on treatment-analyses with the Student’s t-test for variables with a normal distribution and the Mann–Whitney U-test otherwise.
Results
Eighty-one neonates who met the inclusion criteria participated in the study. Five neonates died before the term age, two in the citrate group and three in the control group. In the citrate group a boy born at 29 weeks died at 1 month because of an Enterobacter cloaca sepsis. A girl born at 27 weeks with a birth weight of 745 g died at the age of 10 days, when intensive care treatment was withdrawn, because of severe cerebral damage. Neither had started citrate therapy by the time of death, since they had received less than 24 ml/kg/day enteral feeding. In the control group all three neonates died because of necrotizing enterocolitis. One patient in the citrate group withdrew from the study after 4 days of treatment, without any side effects. An additional patient in the control group developed Candida sepsis, a complication precluding accurate assessment of the kidneys since both Candidiasis (fungal balls) and calcifications could increase renal echogenicity. These patients were not evaluated.
Patient characteristics of the citrate group (n=38) and the control group (n=36) are shown in Table 1. Citrate was administered for a mean of 43.8 (SD 20) days and the reason for interrupting the supplementation was <24 ml/kg/day enteral feeding. Figures 1, 2 and 3 show the results of urine studies for the citrate group and the control group. After one week citrate administration urinary calcium/citrate (Fig. 1) was significantly lower and urinary citrate/creatinine (Fig. 2) and urinary pH (Fig. 3) was significantly higher in the citrate group. There was no difference in urinary calcium/creatinine ratio, oxalate/creatinine ratio and magnesium/creatinine ratio between the two groups. The prevalence of NC was not significantly lower in patients with citrate administration than in control patients (P=0.37, Table 1).
We did not observe a difference in serum calcium and serum creatinine between the citrate group and the control group at day 7, 28 and at term. At day 28 mean serum calcium was 2.46 (0.17) mmol/l in the citrate group vs 2.51 (0.13) mmol/l in the control group and mean serum creatinine was 44 (8) μmol/l vs 48 (17) μmol/l. Treatment with furosemide, thiazide, theophylline and caffeine did not differ significantly between the two groups, neither in the number of treated patients nor in the duration of treatment. The number of patients treated with corticosteroids was significantly higher in the control group than in the citrate group (28% vs 6%, P<0.02). There was no difference in the duration of treatment with corticosteroids.
There were no apparent adverse effects of citrate administration. The patients who received citrate did not suffer more from diarrhea, retention of feeding or vomiting. We found no clinical significant difference in acid-base balance between the patients treated with citrate and control patients (Fig. 4).
Discussion
NC occurs frequently in preterm neonates. Some patients suffer from nephrolithiasis with obstruction of the urinary tract [3, 9] and later in life high blood pressure and impaired renal function may occur more frequently in children with NC than in healthy children [10–12]. We performed the first randomized controlled trial with citrate supplementation to prevent development of NC in premature neonates.
Urine from preterm neonates in the first weeks of life is highly supersaturated and has a defective ability to inhibit calcium oxalate crystal agglomeration. This ability improves with age and is citrate mediated [14]. In a previous study we found a high excretion of calcium and low excretion of citrate in preterm neonates [13]. Sikora et al. [22] also showed low citrate excretion in very low birth weight neonates in the first weeks of life, especially in those <1,000 g.
The first aim of the study was to try to increase citrate excretion. Citrate is freely filtered in the glomerulus. There is no evidence of tubular secretion, thus the excretion is dependent on uptake from the filtrate which mainly takes place in the proximal tubule. Citrate is taken up from the filtrate as divalent anion (citrate2−) with a sodium dependent co-transporter NaDC-1(Na+-decarboxylate co-transporter). At the peritubular space citrate is taken up as trivalent anion (citrate3−) via a co-transporter with sodium. In order to keep the sodium level intracellular low, sodium is transported to the peritubular space by Na+/K+-ATPase. [25]. Therefore, less citrate will be excreted when citrate supplementation is combined with sodium in stead of potassium. In adults it has been demonstrated that potassium citrate supplementation is to be more effective than sodium citrate [26]. Furthermore calcium excretion is linked to sodium excretion; therefore, high sodium intake gives higher calcium excretion. Nevertheless, we chose to use sodium citrate out of concern for the development of hyperkalemia in small neonates on potassium supplementation with low glomerular filtration rates. Even with the use of sodium citrate we demonstrated an increase of citrate excretion, but with careful monitoring of serum potassium higher urinary citrate excretion might be reasonable.
Systemic acidosis has an important effect on citrate excretion by conversion of citrate3− into citrate2− and thereby increasing the availability of transportable divalent citrate, resulting in a decrease of citrate excretion [25]. In our study we only found a higher pH at day 14 in the treated group. We demonstrate an increase of urinary pH after citrate administration in preterm neonates. A higher urinary pH has a decreasing effect on calcium-oxalate crystallization [19].
The excretion of calcium and citrate are associated, because calcium forms complexes with divalent citrate. Since citrate is transported by NADC-1 only as a free ion, complexes with calcium decrease the citrate uptake and increase, therefore, the excretion of citrate [25]. Citrate has an important stone inhibiting effect because the complexes formed with calcium are more soluble than calcium oxalate and calcium phosphate. Besides, citrate directly inhibits the crystallization process [14, 27, 28]. Our study showed lower U-calcium/citrate ratio in the citrate group compared with the control group and it would be interesting to measure if citrate supplementation has a positive effect on the ability to inhibit the agglomeration.
The mechanism by which citrate excretion is low in preterm neonates is not exactly known, but it appears that with maturation of the neonates, citrate excretion climbs to “normal values” at the term age [29]. In our study we found equal values for citrate excretion and pH in the treated and non-treated groups at term. Therefore, we assume that preventive citrate supplementation will only be effective when administered before the term age.
The second aim of the study was to try to prevent the development of NC in preterm neonates. No significant difference in the incidence of NC was found between the two groups in this small population with the use of sodium citrate in this dosage. The dose of citrate administered in this study is based on studies with citrate therapy in children. The dose used in children ranges between 0.1–0.15 g/kg/day (=0.34–0.51 mmol/kg/day) [24, 30]. The fact that the number of patients treated with corticosteroids was higher in the control group than in the treated group might also influence the incidence of NC, as corticosteroids are known to increase calcium excretion [13, 31–33].
Adverse reactions of citrate therapy described in adults are minor gastro-intestinal effects like diarrhea, indigestion, nausea and metabolic alkalosis [17, 18]. In our study there were no apparent adverse effects of citrate administration.
In conclusion, this study demonstrates that sodium citrate administration (0.52 mmol/kg/day) to preterm neonates in the first weeks of life is safe, increases urinary citrate excretion and decreases the U-calcium/citrate ratio, but does not significantly decrease the prevalence of NC. Future studies will be needed to evaluate if treatment with potassium citrate or a higher dose of sodium citrate is equally safe and more effective in the prevention of NC.
References
Jacinto JS, Modanlou HD, Crade M, Strauss AA, Bosu SK (1988) Renal calcification incidence in very low birth weight infants. Pediatrics 81:31–35
Short A, Cooke RW (1991) The incidence of renal calcification in preterm infants. Arch Dis Child 66:412–417
Downing GJ, Egelhoff JC, Daily DK, Alon U (1991) Furosemide-related renal calcifications in the premature infant. A longitudinal ultrasonographic study. Pediatr Radiol 21:563–565
Karlowicz MG, Katz ME, Adelman RD, Solhaug MJ (1993) Nephrocalcinosis in very low birth weight neonates: family history of kidney stones and ethnicity as independent risk factors. J Pediatr 122:635–638
Schell-Feith EA, Holscher HC, Zonderland HM, Kist-van Holthe JE, Conneman HN, van Zwieten PH, Brand R, van der Heijden AJ (2000) Ultrasonic features of nephrocalcinosis in preterm neonates. Br J Radiol 73:1185–1191
Saarela T, Vaarala A, Lanning P, Koivisto M (1999) Incidence, ultrasonic patterns and resolution of nephrocalcinosis in very low birthweight infants. Acta Paediatr 88:655–660
Narendra A, White MP, Rolton HA, Alloub ZI, Wilkinson G, McColl JH, Beattie J (2001) Nephrocalcinosis in preterm babies. Arch Dis Child Fetal Neonatal Ed 85:F207–F213
Hein G, Richter D, Manz F, Weitzel D, Kalhoff H (2004) Development of nephrocalcinosis in very low birth weight infants. Pediatr Nephrol 19:616–620
Alpert SA, Noe HN (2004) Furosemide nephrolithiasis causing ureteral obstruction and urinoma in a preterm neonate. Urology 64:589
Ezzedeen F, Adelman RD, Ahlfors CE (1988) Renal calcification in preterm infants: pathophysiology and long- term sequelae. J Pediatr 113:532–539
Downing GJ, Egelhoff JC, Daily DK, Thomas MK, Alon U (1992) Kidney function in very low birth weight infants with furosemide- related renal calcifications at ages 1 to 2 years. J Pediatr 120:599–604
Schell-Feith EA, Kist-van Holthe JE, Van Zwieten PH, Zonderland HM, Holscher HC, Swinkels DW, Brand R, Berger HM, van der Heijden BJ (2003) Preterm neonates with nephrocalcinosis: natural course and renal function. Pediatr Nephrol 18:1102–1108
Schell-Feith EA, Kist-van Holthe JE, Conneman N, Van Zwieten PH, Holscher HC, Zonderland HM, Brand R, van der Heijden BJ (2000) Etiology of nephrocalcinosis in preterm neonates: association of nutritional intake and urinary parameters. Kidney Int 58:2102–2110
Schell-Feith EA, Que I, Kok DJ, Kist-van Holthe JE, Kuhler E, Brand R, Papapoulos SE, van der Heijden BJ (2001) Modulation of calcium oxalate monohydrate crystallization kinetics by urine of preterm neonates. Am J Kidney Dis 38:1229–1234
Pak CY, Fuller C, Sakhaee K, Preminger GM, Britton F (1985) Long-term treatment of calcium nephrolithiasis with potassium citrate. J Urol 134:11–19
Hauser W, Frick J, Kunit G (1990) Alkali citrate for preventing recurrence of calcium oxalate stones. Eur Urol 17:248–251
Barcelo P, Wuhl O, Servitge E, Rousaud A, Pak CY (1993) Randomized double-blind study of potassium citrate in idiopathic hypocitraturic calcium nephrolithiasis. J Urol 150:1761–1764
Pak CY (1997) Southwestern Internal Medicine Conference: medical management of nephrolithiasis-a new, simplified approach for general practice. Am J Med Sci 313:215–219
Berg C, Larsson L, Tiselius HG (1992) The effects of a single evening dose of alkaline citrate on urine composition and calcium stone formation. J Urol 148:979–985
Adams ND, Rowe JC (1992) Nephrocalcinosis. Clin Perinatol 19:179–195
Al Qadreh A, Athanasopoulou H, Voskaki I (1992) Inhibitors of stone formation in hypercalciuric children with and without stone disease. Eur Urol 21:227–230
Sikora P, Roth B, Kribs A, Michalk DV, Hesse A, Hoppe B (2003) Hypocitraturia is one of the major risk factors for nephrocalcinosis in very low birth weight (VLBW) infants. Kidney Int 63:2194–2199
Pope JC, Trusler LA, Klein AM, Walsh WF, Yared A, Brock JW (1996) The natural history of nephrocalcinosis in premature infants treated with loop diuretics. J Urol 156:709–712
Leumann E, Hoppe B, Neuhaus T (1993) Management of primary hyperoxaluria: efficacy of oral citrate administration. Pediatr Nephrol 7:207–211
Unwin RJ, Capasso G, Shirley DG (2004) An overview of divalent cation and citrate handling by the kidney. Nephron Physiol 98:15–20
Preminger GM, Sakhaee K, Pak CY (1988) Alkali action on the urinary crystallization of calcium salts: contrasting responses to sodium citrate and potassium citrate. J Urol 139:240–242
Hojgaard I, Tiselius HG (1998) The effects of citrate and urinary macromolecules on the aggregation of hydroxyapatite crystals in solutions with a composition similar to that in the distal tubule. Urol Res 26:89–95
Kok DJ, Papapoulos SE, Blomen LJ, Bijvoet OL (1988) Modulation of calcium oxalate monohydrate crystallization kinetics in vitro. Kidney Int 34:346–350
White MP, Aladangady N, Rolton HA, McColl JH, Beattie J (2005) Urinary citrate in preterm and term babies. Early Hum Dev 81:319–323
Leumann E, Hoppe B, Neuhaus T, Blau N (1995) Efficacy of oral citrate administration in primary hyperoxaluria. Nephrol Dial Transplant 10 Suppl 8:14–16
Stapleton FB, McKay CP, Noe HN (1987) Urolithiasis in children: the role of hypercalciuria. Pediatr Ann 16:980–981,984–992
Kamitsuka MD, Williams MA, Nyberg DA, Fox KA, Lee DL, Hickok D (1995) Renal calcification: a complication of dexamethasone therapy in preterm infants with bronchopulmonary dysplasia. J Perinatol 15:359–363
Sonntag J, Gaude M (1998) Effect of dexamethasone and spironolactone therapy in calcium and phosphate homeostasis in premature infants with a birth weight under 1,500 g. Klin Padiatr 210:354–357
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schell-Feith, E.A., Moerdijk, A., van Zwieten, P.H.T. et al. Does citrate prevent nephrocalcinosis in preterm neonates?. Pediatr Nephrol 21, 1830–1836 (2006). https://doi.org/10.1007/s00467-006-0274-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-006-0274-4