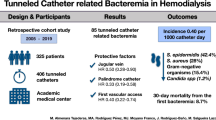
Abstract
Tunneled central venous catheters are often used in children on chronic hemodialysis. This study was done to evaluate the spectrum of catheter-related bacteremia (CRB) and to determine predictors of recurrent CRB in children on hemodialysis. Chart review was performed in 59 children from a pediatric dialysis unit with chronic, tunneled, cuffed hemodialysis catheters between January 1999 and December 2003. CRB was diagnosed in 48 of 59 (81%) patients. The incidence of CRB was 4.8/1,000 catheter days. Overall catheter survival (290±216 days) was significantly longer than infection-free catheter survival (210±167 days, p<0.05). Organisms isolated were gram-positive in 67%, gram-negative in 14%, and polymicrobial in 19%. Systemic antibiotics cleared CRB in 34% and an additional 23% cleared with the inclusion of antibiotic-heparin locks; 43% required catheter exchange. There was a significant likelihood of early catheter exchange with polymicrobial CRB (p<0.01). Catheter loss occurred from infection in 63%. Risk factors for CRB included young age (<10 years) and presence of human immunodeficiency virus (HIV) infection. Patients with >2 initial positive blood cultures (p<0.0001) had a significantly higher rate of recurrence after 6 weeks of initial treatment. In conclusion, CRB remains a major determinant of catheter loss. However, overall catheter survival is longer than infection-free catheter survival, suggesting that systemic antibiotics with antibiotic-heparin locks should be the initial step in the management of CRB and this approach may salvage some catheters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tunneled cuffed central venous catheters are frequently used in children on long-term hemodialysis [1]. Permanent vascular access is limited by relative difficulties in creating and maintaining patency of arteriovenous (AV) fistulas and AV grafts in small children [1, 2]. There is a center-dependent variability in technique and success rate [3, 4]. Bacteremia, thrombosis, and catheter malfunction are some of the complications encountered with the use of central venous catheters. These complications increase patient morbidity and shorten catheter survival. Previous studies have reported the frequency of catheter-related bacteremia (CRB) to be around 1.2–4 CRB/1,000 catheter days [5–8]. In addition to sepsis, CRB can result in metastatic infections like osteomyelitis, epidural abscesses, or infective endocarditis [5]. Studies in children have been limited by their small numbers and short-term outcome [5–10]. In this study we evaluate the spectrum of CRB and identify factors predictive of recurrent CRB in children on chronic hemodialysis. We also analyzed short- and long-term outcomes of treatment of CRB with systemic antibiotics (with and without heparin-antibiotic locks) and catheter replacement.
Patients and methods
Our Institutional Review Board approved this study. Retrospective chart review was performed in 70 children who were chronically dialyzed in the pediatric hemodialysis unit at the University of Miami/Holtz Children’s Center from January 1999 to December 2003. All consecutive patients were included in the study. Those on temporary hemodialysis were excluded. Tunneled, cuffed double-lumen hemodialysis catheters (Medcomp®) were used for vascular access and placed by the pediatric surgeon in children <15 kg and by the interventional radiologist in the others. The size and the length of the catheters were based on the patient’s size. Patients were dialyzed 3–4 times per week, on hollow-fiber dialyzers appropriate for their body size with Cobe® or Baxter® hemodialysis machines. A standard bicarbonate bath was used as dialysate. Intravenous antibiotics, vitamin D analogs, erythropoietin, and intravenous iron supplements were given towards the end of dialysis as needed. Dialysis efficiency was monitored on a monthly basis and the goal for Kt/V was >1.2.
Catheter care and use
Hemodialysis catheters were handled only during dialysis sessions with no intervention between treatments. Since the year 2001, our protocol for exit site care was to clean with betadine solution, apply a Biopatch® (chlorhexidine-impregnated dressing), and cover with a transparent dressing (Tegaderm)®. This process was repeated once a week. At the end of each dialysis session, each port of the catheter was filled with 5,000 U/ml of heparin solution according to the volume of the ports.
Protocol for catheter-related bacteremia
Definitions applicable to the protocol
-
1.
Exit site infection
Presence of purulent discharge, swelling, erythema, and tenderness at the exit site.
-
2.
Catheter-related bacteremia
Occurrence of a positive blood culture from the catheter with or without a peripheral blood culture in the presence of systemic symptoms with no other source of infection identified. Recurrent CRB was defined as occurrence of ≥1 CRB after 6 weeks of initial treatment.
-
3.
Polymicrobial infection
The growth of at least two or more microorganisms in the first or sequential blood cultures during the same CRB.
Initial identification and management
Blood cultures were obtained from both ports of the catheter if the patient had fever, chills, weakness, or emesis. Patients were examined to determine a source for their fever and, if catheter-related bacteremia was suspected, intravenous antibiotics (vancomycin and levofloxacin) were empirically administered to treat both gram-positive and gram-negative organisms. The treatment regimen was later narrowed according to sensitivities. Patients with positive blood cultures were treated for 3 weeks or until at least two blood cultures taken 1 week apart were negative. Since the year 2003, patients with persistently positive blood cultures who were symptomatic initially but asymptomatic after the first 24–48 h of systemic antibiotic treatment were treated with antibiotic-heparin locks in an attempt to eradicate bacterial colonization in the catheter. The antibiotics used in the lock were either vancomycin or tobramycin (for gram-negative and polymicrobial) depending on the sensitivity of the organism. The concentration used for each was 5 mg/ml with 5,000 U/ml of heparin in each port. The concentration was based on literature reports and compatibility testing by the pharmacist [11–14]. The solution was prepared at the bedside by the nurse.
Patients who continued to be symptomatic after 24–48 h of systemic antibiotic treatment had their catheters exchanged. Surveillance blood cultures were not done.
Catheter removal
Catheters were removed if there was malfunction, cuff extrusion or catheter breakage, relapsing infection, growth of fungus or methicillin-resistant Staphylococcus aureus, or persistent fever beyond 3 days of therapy. Patients who had ongoing evidence of sepsis had their catheters removed and a temporary catheter placed for hemodialysis until their symptoms resolved. Two different methods of replacement were used:
-
1.
Removing the infected catheter and placing another one through a new tunnel.
-
2.
Exchanging the catheter with a guide wire using the same tunnel (WGE). The method of replacement was decided according to the severity of the symptoms and the experience of the radiologist or surgeon.
Antibiotic treatment regimens
The antibiotic treatment regimens were categorized as follows:
-
1.
Parenteral antibiotic therapy alone
-
2.
Parenteral antibiotic followed by luminal installation of antibiotic-heparin lock if the patient had persistently positive blood cultures without any symptoms
-
3.
Systemic antibiotics followed by wire-guided exchange
-
4.
Systemic antibiotics followed by replacement of the catheter at a different site
Drug levels of vancomycin and/or aminoglycoside (if applicable) were obtained in all patients prior to dialysis and this was used as a guide to give supplemental doses. Data were obtained on serum albumin, C-reactive protein (CRP), serum hemoglobin concentration, ferritin levels, and number of doses of parenteral iron. Echocardiograms were obtained as part of the annual routine or, more frequently, if there was a medical indication such as dilated cardiomyopathy or suspected intracardiac thrombus. Echocardiography was performed in the course of a CRB if blood cultures remained positive despite appropriate treatment.
Statistical methods
Descriptive statistics were used to summarize data. P values of less than 0.05 were considered significant. All results are expressed as the mean±standard deviation (SD). Statistical analyses were performed using the Graph Pad® statistical software and SAS. Fisher’s test was used to assess the risk of CRB with age and to determine odds of treatment failure with bacterial etiology, number of positive blood cultures, and modality of treatment. Infection-free and overall catheter survival as well as biochemical parameters between catheters with and without recurrent CRB were compared with Student’s t-test. Prevalence of CRB according to primary etiology of end-stage renal disease (ESRD) was analyzed with analysis of variance (ANOVA). The association between biochemical parameters and recurrence at 6 weeks was analyzed by stepwise logistic regression.
The short-term outcome endpoint was defined as having at least two negative blood cultures 1 week apart after the initial CRB. Failure was defined as persistence of positive blood cultures despite antibiotic treatment. The outcome at 6 weeks was the long-term endpoint where recurrence was defined as the occurrence of another CRB. No surveillance blood cultures were obtained. The number of days before the first CRB was a measure of infection-free catheter survival and the number of days before the actual catheter removal was a measure of overall catheter survival.
Results
The mean age (±SD) of the patients was 13.9±4.6 years. There were 30 males and 40 females of whom 39 were Black, 23 Hispanic, and 8 White. Of the 70 children, 14 (20%) had AV fistulas or grafts at initiation or during the course of the study; 59 (84%) had chronic hemodialysis catheters (including 4 who later had fistulas or grafts). There were 138 right internal jugular, 26 left internal jugular, 9 right subclavian, and 2 left femoral catheters. All first catheters were right internal jugular.
The average time on hemodialysis with a tunneled cuffed catheter was 676±437 days. The primary etiology for ESRD was obstructive uropathy/dysplasia in 17, vasculitis/lupus nephritis in 10, chronic glomerulonephritis in 20, human immunodeficiency virus (HIV)-associated nephropathy in 8, other diseases in 8, and unknown in 7.
Catheter-related bacteremia was diagnosed in 48 of 59 (81%) patients with a total of 188 CRB and a total observation of 38,888 catheter days. Eleven patients (19%) had no CRB during the study period. Gram-positive bacteremia was significantly more prevalent than gram-negative bacteremia, with an average occurrence of 67% over the 5 years. Figures 1 and 2 describe the spectrum of organisms and their resistance patterns. Gram-positive isolates were resistant to oxacillin in 76.4 and sensitive to vancomycin in 99.3% of cases. Tobramycin resistance was demonstrated in 22% of gram-negative CRB. This resistance increased to 72% in polymicrobial infections. Of all gram-negative isolates, 100% were sensitive to levofloxacin. No polymicrobial infections were encountered with first catheter infections where gram-negative organisms constituted 17%. Infection with Pseudomonas occurred in four blood cultures. Infection cleared with systemic antibiotic therapy and antibiotic-heparin lock in two children; catheter exchange was required in two.
The rate of CRB was 4.8 episodes/1,000 catheter days. The rate of catheter removal as a consequence of CRB was 1.9/1,000 catheter days. The infection-free survivals of subsequent catheters were significantly shorter than that of the first catheter (p<0.05). The rate of exit site infections was 0.6 episodes/1,000 catheter days. The organisms isolated from the exit site correlated with only four CRBs. There was a significant decrease in the number of exit site infections in the last 2 years of the study period attributable to the use of the chlorhexidine-impregnated patch at the exit site (p<0.05). However, this did not change the rate of occurrence of CRB. Figures 3 and 4 show the treatment regimens and recurrence rate at 6 weeks.
Type of CRB, initial treatment, and recurrence at 6 weeks. Initial treatment consisted of systemic antibiotics alone (AB) or systemic antibiotics followed by the addition of antibiotic-heparin lock in 48–72 h when blood cultures were persistently positive (AB&L). The introduction of antibiotic-heparin locks cleared the initial bacteremia in an additional 23%. However, the rate of recurrence of CRB at 6 weeks was comparable; 50% of recurrences were with the same organism
Type of CRB, initial treatment, and recurrence at 6 weeks. Initial treatment consisted of systemic antibiotics followed by catheter exchange when patients persisted with symptoms of bacteremia. Method of exchange was either wire-guided exchange (WGE) or removal of the catheter with replacement at a different site (RR). Rate of recurrence did not change with treatment modality. Recurrence with the same organism did not occur in RR but occurred in 17% of WGE
The patient’s treatment modality depended on their initial response to systemic antibiotics. Systemic antibiotic therapy cleared the CRB in 64 of 188 (34%) cases; the addition of antibiotic-heparin locks cleared the initial bacteremia in an additional 23%; 81 of 188 (43%) CRB required early catheter exchange (in 2–7 days) in addition to systemic antibiotics. Three patients failed their initial treatment regimen (short-term outcome endpoint). Two of the three died despite removal of the infected catheter. Their cause of death was multifactorial and related to complications from HIV infection.
Gram-positive CRB was more likely to be treated with systemic antibiotics alone [p<0.001, odds ratio: 3.3, 95% confidence interval (CI): 1.6–7]. Children with polymicrobial infections had a greater chance of requiring catheter exchange during initial treatment (p<0.01, odds ratio: -2.7, 95% CI: 1.3-5.8). CRB recurred in 55 of 188 (29%) after 6 weeks of initial therapy (long-term endpoint; Figs. 3 and 4). There was no association between initial treatment modality and recurrence at 6 weeks. Recurrence at 6 weeks was associated with >2 initial positive blood cultures (p<0.0001; Fig. 5). When there was one positive blood culture, recurrence at 6 weeks was less likely with gram-positive CRB as compared to gram-negative CRB (p<0.01, odds ratio: 6.6, 95% CI: 1.5–30). Recurrence with the same organism did not occur if the initial treatment consisted of catheter removal and replacement (p<0.01). There was no association between biochemical parameters and recurrence at 6 weeks. History of previous CRB did not predict the etiology or sensitivities of subsequent CRB.
Blood cultures were done on consecutive dialysis days when CRB was diagnosed, until a negative culture is obtained. Thereafter, it was repeated every week until at least two consecutive blood cultures were negative. Figure 5 demonstrates the significant increase in percent recurrence of CRB at 6 weeks, when the number of initial positive blood cultures is >2; *p<0.0001
Over the 5-year period, there were 175 catheters: 57 were first time and 118 were replaced or exchanged catheters. The most frequent reasons for exchange or replacement of catheters were infection in 63%, catheter malfunction in 32%, and cuff extrusion in 5%. Overall catheter survival was significantly increased when compared to infection-free catheter survival (290±216 days versus 210±167 days, p<0.05). Children with recurrent CRB had shorter catheter survival compared to children with no CRB (234±178 versus 366±216 days, p<0.05). There were 56 hospital admissions for CRB over the 5 years with mean hospital stay of 12.1±12.3 days. Two patients with candida/polymicrobial bacteremia and one patient with an infected thrombus had the longest hospital days (range: 27–37 days).
A total of 275 cardiac echocardiograms were performed on the 60 patients. Six patients were diagnosed with a thrombus at the catheter tip extending into the right atrium. Five of these had recurrent CRB and four patients had bacterial endocarditis including two with a thrombus. However, this was not statistically significant.
Younger age (<10 years) posed a greater risk of CRB occurring at a rate of 5.6/1,000 catheter days (p<0.05, odds ratio: 95% CI: 4.2–7.0). Serum albumin, ferritin, hemoglobin, and CRP were compared in first catheters with ≥2 CRB versus those with <2 CRB and were not significantly different between groups (see Table 1). However, those catheters with <2 CRB had increased mean dose of IV iron when compared to those with ≥2 CRB. Catheters of patients with HIV-associated nephropathy had the highest prevalence of CRB (8.4/1,000 catheter days, p<0.001).
Discussion
In our center, a large proportion of children are dialyzed through central venous catheters due to failure and/or exhaustion of permanent vascular access, small patient size, and inability to initiate or continue peritoneal dialysis. A significant proportion of patients were on hemodialysis for greater than the average period of time due to the nature of their primary disease (HIV, malignancy), inadequate family support, and high panel-reactive antibodies. Experience with long-term management of patients with HIV has steered us away from peritoneal dialysis since they develop fungal peritonitis [15–17]. As a consequence, the total number of catheters involved, cumulative catheter days, and the patient pool makes this study one of the largest and most diverse reported cohorts in children.
Despite observing guidelines set forth by the Center for Disease Control, CRB is a significant problem in hemodialysis units [18]. Catheter survival is greatly influenced by episodes of catheter-related bacteremia, as seen in several studies [5, 7–9]. Marr et al. had results similar to our study and showed that systemic antibiotics can only salvage about 30% of catheters [3]. If one considers the frequency of occurrence of CRB, it would not be feasible to remove catheters with every infection.
This study was not prospective and therefore treatment was not randomized. The initial modality of treatment was dictated by the treating physician’s clinical judgement. Due to the emergence of resistant strains of gram-negative and polymicrobial CRB to tobramycin in our hemodialysis unit, and its potential for ototoxicity, levofloxacin was used as the initial choice for targeting gram-negative organisms. Moreover, there was 100% sensitivity of the gram-negative organisms to levofloxacin. Its use had several advantages including convenient administration every 48 h in the setting of end-stage kidney disease and the potential to treat most gram-positive organisms, thus decreasing the need to use vancomycin for quinolone-susceptible bacteria. Moreover, oral doses were comparable to parenteral doses offering the advantage of using levofloxacin on days off dialysis. Articular damage has been reported in young animals necessitating caution in using quinolones in young children. Studies in children thus far have revealed no permanent articular damage [19].
Gram-positive CRB was more likely to be treated with systemic antibiotics with or without antibiotic-heparin lock solutions, whereas gram-negative and polymicrobial CRB was more likely to be treated with catheter exchange as a consequence of the patient’s initial symptoms. In this study, 43% of CRB required early catheter exchange. However, the recurrence rate at 6 weeks did not change even when catheters were replaced during initial treatment. One of the explanations for recurrent CRB is bacterial adhesion to the catheter surface in a protein matrix, which results in a biofilm that causes intermittent shedding of bacteria into the bloodstream [20–22]. The use of antibiotic-heparin locks may be an important addendum to treatment as it is aimed at the biofilm [11–14]. We were able to increase the success rate of treatment with antibiotics from 34 to 57%, in a select group of patients with persistent culture-positive CRB, when antibiotic-heparin locks were added later. The introduction of antibiotic-heparin locks from the beginning may decrease colonization and increase catheter survival time. This should be further investigated in the pediatric population.
Risk factors for CRB have been identified in other studies and include immune deficiency, high serum ferritin, intravenous iron use, and low serum albumin [23–30]. In our analysis, low serum albumin was associated with recurrent CRB. In contrast to other studies, we observed that mean IV iron dose was higher in those catheters without recurrent CRB despite comparable hemoglobin values. A possible explanation is that aggressive correction of anemia may decrease susceptibility to infection. A risk factor that needs to be taken into consideration is the presence of thrombi at the catheter tip or in the right atrium. Recurrent CRB occurred in five of six catheters with thrombi at the catheter tip, although this was not statistically significant when compared to catheters without thrombi. Endocarditis was the only metastatic infectious complication of CRB in our population. An important risk factor for recurrence of CRB was having >2 positive blood cultures with the initial infection.
In conclusion, in our population, younger age (<10 years), HIV infection, and multiple positive blood cultures were major risk factors for CRB affecting catheter survival. However, our study demonstrates that overall catheter survival is significantly longer than infection-free catheter survival proving that some infected catheters can be salvaged. Moreover, recurrence of CRB at 6 weeks after initial treatment occurs at a comparable rate regardless of initial modality of treatment. Preservation of venous access sites is vital in children, not only to save the vascular access sites, but also prevent unnecessary intervention and anesthesia. However, catheter exchange will still be necessary in patients who have sepsis, metastatic infections, or persistent CRB. Systemic antibiotics should be the initial step in management of CRB. The addition of antibiotic-heparin lock solution may salvage colonized catheters when used in conjunction with systemic antibiotics. There should be a high index of suspicion for central venous and intra-atrial thrombi in the presence of recurrent CRB.
Pediatric hemodialysis patients are more likely to require central venous catheters for temporary or permanent vascular access [31, 32]. Although technical improvements in vascular access surgery will allow AV fistulas or grafts in some patients, central venous catheters will remain a necessary mode of vascular access in many children, especially the very young. Therefore, further research needs to be done to devise methods to prevent CRB and to treat it more effectively.
References
Ramage IJ, Bailie A, Tyerman KS, McColl JH, Pollard SG, Fitzpatrick MM (2005) Vascular access survival in children and young adults receiving long-term hemodialysis. Am J Kidney Dis 45:708–715
Lumsden AB, MacDonald MJ, Allen RC, Dodson TF (1994) Hemodialysis access in the pediatric patient population. Am J Surg 168:197–201
Bourquelot P, Raynaud F, Pirozzi N (2003) Microsurgery in children for creation of arteriovenous fistulas in renal and non-renal diseases. Ther Apher Dial 7:498–503
Gradman WS, Lerner G, Mentser M, Rodriguez H, Kamil ES (2005) Experience with autogenous arteriovenous access for hemodialysis in children and adolescents. Ann Vasc Surg 19:609–612
Marr KA, Sexton D, Conlon PJ, Corey GR, Schwab SJ, Kirkland KB (1997) Catheter-related bacteremia and outcome of attempted catheter salvage in patients undergoing hemodialysis. Ann Intern Med 127:275–280
Goldstein SL, Macierowski CT, Jabs K (1997) Hemodialysis catheter survival and complications in children and adolescents. Pediatr Nephrol 11:74–77
Sharma A, Zilleruelo G, Abitbol C, Montane B, Strauss J (1999) Survival and complications of cuffed central venous catheters in children and young adults on chronic hemodialysis. Pediatr Nephrol 13:245–248
Hymes LC, Warshaw BL, Clowers B, Newsome P, Keyserling HL (1996) Bacteremia in a pediatric hemodialysis unit secondary to Enterococcus fecalis. Pediatr Nephrol 10:55–57
Chawla PG, Nevins TE (2000) Management of hemodialysis catheter-related bacteremia—a 10-year experience. Pediatr Nephrol 14:198–202
Paglialonga F, Esposito S, Edefonti A, Principi N (2004) Catheter-related infections in children treated with hemodialysis. Pediatr Nephrol 19:1324–1333
Johnson DC, Johnson FL, Goldman S (1994) Preliminary results treating persistent central venous catheter infections with the antibiotic lock technique in pediatric patients. Pediatr Infect Dis J 13:930–931
Capdevila JA, Segarra A, Planes AM, Ramirez-Arellano M, Pahissa A, Piera L, Martinez-Vazquez JM (1993) Successful treatment of haemodialysis catheter-related sepsis without catheter removal. Nephrol Dial Transplant 8:231–234
Bailey E, Berry N, Cheesbrough JS (2002) Antimicrobial lock therapy for catheter-related bacteremia among patients on maintenance haemodialysis. J Antimicrob Chemother 50:615–617
Krishnasami Z, Carlton D, Bimbo L, Taylor ME, Balkovetz DF, Barker J, Allon M (2002) Management of hemodialysis catheter-related bacteremia with an adjunctive lock solution. Kidney Int 61:1136–1142
Zilleruelo G, Strauss J (1994) Management of ESRD in children with AIDS nephropathy. Semin Dial 7:454–460
Zilleruelo G, Abitbol CL, Hubsch H, Montane B, Chandar J, Strauss J (2003) Dialysis treatment of pediatric patients infected with the human immunodeficiency virus (abstract W864). Nephrol Dial Transplant 18(Suppl 4):826
Abitbol CL, Friedman LB, Zilleruelo G (2005) Renal manifestations of sexually transmitted diseases: sexually transmitted diseases and the kidney. Adolesc Med Clin 16:45–65
O’Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, Masur H, McCormick RD, Mermel LA, Pearson ML, Raad II, Randolph A, Weinstein RA, Healthcare Infection Control Practices Advisory Committee (2002) Guidelines for the prevention of intravascular catheter-related infections. Infect Control Hosp Epidemiol 23:759–769
Chalumeau M, Tonnelier S, d’Athis P, Treluyer JM, Gendrel D, Breat G, Pons G, The Pediatric Fluoroquinolone Safety Study Investigators (2003) Flouroquinolone safety in pediatric patients: a prospective, multicenter, comparative cohort study in France. Pediatrics 111:e714–e719
Dasgupta MK (2002) Biofilms and infection in dialysis patients. Semin Dial 15:338–346
Costerton JW, Philip SS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322
Donlan RM (2001) Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis 33:1387–1392
Hoen B, Kessler M, Hestin D, Mayeux D (1995) Risk factors for bacterial infections in chronic hemodialysis adult patients: a multicenter prospective survey. Nephrol Dial Transplant 10:377–381
Jean G, Charra B, Chazot C, Vanel T, Terrat JC, Hurot JM, Laurent G (2002) Risk factor analysis for long-term tunneled dialysis catheter-related bacteremias. Nephron 91:399–405
Kaplowitz LG, Comstock JA, Landwehr DM (1988) A prospective study of infections in hemodialysis patients: patient hygiene and other risk factors for infection. Infect Control Hosp Epidemiol 9:534–541
Brewster UC, Coca SG, Reilly RF, Perazella MA (2005) Effect of intravenous iron on haemodialysis catheter microbial colonization and blood-borne infection. Nephrology (Carlton) 10:124–128
Sirken G, Raja R, Rizkala AR (2004) Identification of infectious risk factors in maintenance hemodialysis patients: the role of intravenous iron. J Am Soc Nephrol 15:627A
Churchill DN, Taylor DW, Cook RJ (1992) Canadian hemodialysis morbidity study. Am J Kidney Dis 19:214–234
Powe NR, Jaar B, Furth SL (1999) Septicemia in dialysis patients: incidence, risk factors, and prognosis. Kidney Int 55:1081–1090
Tanriover B, Carlton D, Saddekni S, Hamrick K, Oser R, Westfall A, Allon M (2000) Bacteremia associated with tunneled dialysis catheters: comparison of two treatment strategies. Kidney Int 57:2151–2155
2003 Annual Report (2004) ESRD Clinical Performance Measures Project. Opportunities to improve care for adult in-center hemodialysis, adult peritoneal dialysis, and pediatric in-center hemodialysis patients. Am J Kidney Dis 44 (Suppl 2): 44–52
Neu MA, Ho PL, McDonald RA, Warady BA (2002) Chronic dialysis in children and adolescents. The 2001 NAPRTCS Annual Report. Pediatr Nephrol 17:656–663
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Onder, A.M., Chandar, J., Coakley, S. et al. Predictors and outcome of catheter-related bacteremia in children on chronic hemodialysis. Pediatr Nephrol 21, 1452–1458 (2006). https://doi.org/10.1007/s00467-006-0130-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-006-0130-6