Abstract
The finding of scintigraphic renal defects in children with febrile urinary tract infection (UTI) even in the absence of vesicoureteric reflux (VUR) has led to the conclusion that VUR is a weak predictor of renal defects in these patients. We used isotopic cystography (IC) for diagnosis of VUR in children with febrile UTI. Dimercaptosuccinic acid renal scintigraphy was performed 6 months after cure of the last UTI. Renal defects were defined by the finding of focal defects of radionuclide uptake and/or by a split renal function <43%. The study included 206 children with primary VUR and 77 without VUR. Among the subjects with and without VUR, respectively, renal defects were found in 40 and 6% (p=0.0001), focal uptake defects in 33 and 5% (p=0.0001) and split renal function <43% in 26 and 5% (p=0.0001). Permanent renal defects in children with febrile UTI are closely associated with VUR. The possibility that a child will have permanent renal defects can reasonably be ruled out on the basis of the absence of VUR by IC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Renal parenchymal defects due to urinary tract infection (UTI) are one of the major causes of acquired renal damage. The inflammatory changes seen associated with acute pyelonephritis are reversible but, in some cases, result in renal defects which can lead to hypertension and chronic renal failure [1–3] as well as to complications during pregnancy and pre-eclampsia [4]. Therefore, establishing the occurrence of permanent renal parenchymal defects after acute pyelonephritis may be important for long-term prognosis. Dimercaptosuccinic acid (DMSA) renal scintigraphy is the most sensitive tool for diagnosis of acute pyelonephritis and permanent renal defects [5]. The incidence of renal defects correlates inversely with the time interval between pyelonephritis and the scintigraphic study and stabilizes some 4–6 months following acute disease [6].
Vesicoureteric reflux (VUR) has long been considered an absolute prerequisite for renal defects but, in the last decade, this belief has been questioned. In particular, a recent meta-analysis by Gordon et al. [7] has shown that many infants and children with scintigraphic signs of renal defects, detected—in most cases—within 6 weeks of febrile UTI, did not have demonstrable VUR. This has strengthened the doubts about the role of VUR in the genesis of UTI-related renal defects and has led to the conclusion that VUR is a weak predictor of renal defects in children hospitalized with UTI and that cystography should not be used as a screening tool to exclude renal defects [7].
It is known that VUR may be intermittent and that it may be missed with the conventional X-ray voiding cystourethrography (VCU) [8–10]. Actually, the detection of VUR with the classic one-filling VCU is significantly enhanced with cyclic VCU [8, 9] and is further enhanced with isotopic cystography (IC) [10]. The studies reporting comparable prevalences of renal defects in subjects with and without VUR used the classic one-filling VCU to detect reflux [7]. Hence, the question arises of whether renal defects were, in fact, exclusively associated with reflux, because the subjects with renal defects and no VUR could have had an occult reflux undetected with the conventional study.
The present study attempts to answer that question by analysing the occurrence of permanent scintigraphic renal defects in infants and children who had febrile UTI in which VUR was detected with IC which represents, in our [10] and others’ [11–15] experience, the most sensitive of the current diagnostic tools.
Patients and methods
We report a prospective observational study of unselected children referred with fever who were diagnosed to have UTI. They were seen in our setting following the start of a diagnostic protocol including IC for the first-step diagnosis of VUR [10] and later DMSA renal scintigraphy to detect permanent renal defects.
By January 2000 to January 2004, all children referred at any age with fever (≥38.3°C) who were diagnosed by us to have UTI underwent micturition IC and renal ultrasound. IC was performed as previously reported [10] after 3–6 weeks of sterile urine. Boys with VUR diagnosed by IC also underwent X-ray VCU to detect urethral valves. Subjects with secondary VUR (neurogenic bladder, urethral valves, duplex systems, ureterocele) were excluded from the study. The diagnostic protocol included DMSA renal scan 6 months after the last UTI in all patients with VUR and only in those patients without VUR who were considered at higher risk for renal abnormality, i.e. with at least two febrile UTI or one febrile UTI whose antibacterial treatment was started ≥4 days after the onset of fever. DMSA renal scintigraphy was performed between June 2000 and June 2004. Written informed consent for the diagnostic procedures was obtained from the children’s parents.
Laboratory and imaging studies
A diagnosis of UTI required significant bacteriuria, that is, in one midstream sample or in two bag urine samples ≥105 colony-forming units/ml of a single species or ≥104 in urine obtained by bladder catheterisation. Creatinine clearance (CCr) was calculated by patient height and serum creatinine according to the Schwartz equations [16] at diagnosis of UTI in all patients and 3 months after the cure of UTI in those with CCr values less than −2 standard deviations (SD) of average normal values at first determination. We considered values to be abnormal when they remained less than −2 SD of average normal values [16] even 3 months after pyelonephritis was cured. Blood pressure was measured by Dinamap Critikon according to the criteria of the Task Force for Blood Pressure Control in Children and Adolescents [17]. Hypertension was considered as blood pressure above the 95th percentile for gender and age [17].
Reflux seen at IC was graded as mild (reflux to ureter or renal pelvis), moderate (reflux to renal pelvis with mild to moderate dilatation) and severe (distended redundant collecting system associated with ureteral dilatation). Renal scintigraphy was performed 6 months after cure of the last UTI; 0.5 MBq/kg body weight (minimum 10 MBq) of technetium 99-dimercaptosuccinic acid was administered intravenously. Two hours later, anterior, posterior and left and right oblique posterior planar images were obtained by a gamma camera (Siemens Orbiter 75-, Erlangen, Germany) equipped with a Maxdelta computer system. The percentage of tracer uptake by both kidneys was obtained. Computer images were analysed visually for evidence of cortical loss and photopenia consistent with cortical scarring. Renal parenchymal defects were defined as the presence of focal or multifocal defects of uptake or/and as a split renal isotope uptake of less than 43%. In the analysis of scintigraphic findings, five main categories of renal parenchymal defects were taken into account: (1) split function <43% only, (2) focal defects only, (3) combined defects, i.e. 1+2, (4) split function <43% in total and (5) focal defects in total. Moreover, in the attempt to identify as best we could the subjects with post-infectious renal defects, we arbitrarily considered a further category of most likely acquired defects, which included the subjects with focal defects only as well as those with combined abnormality, having a split function above 30% of total and a normal CCr. This was done, because in a more severely damaged kidney it may be more difficult to discriminate between a congenitally hypodysplastic kidney and one with serious post-infectious damage. Cystographic and renal scintigraphic tests were read blindly always by the same observer. When reading the renal DMSA scan, the observer was unaware of the results of the IC performed 6 months before.
Patient selection
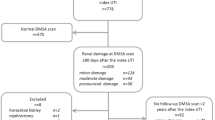
During the study period, 388 patients with febrile UTI underwent IC. Five of them had secondary VUR, 235 had primary VUR and 148 had no VUR.
Of the 235 with primary VUR, 25 subjects had no DMSA scan performed because they were firstly seen by us—and underwent IC—<6 months after their UTI, i.e. before they would have had a DMSA scan performed according to our protocol, and 3 because of denied consent to the procedure or loss to follow-up. Another patient with primary VUR who underwent a DMSA scan was excluded because of renal ptosis which made morphologic and functional evaluation of the kidney unreliable. This led to a final sample of 206 children with primary VUR studied (Table 1).
Of the 148 children without VUR, 53 did not have and 95 had DMSA study indicated by our protocol. Of the 95, 8 had no DMSA study performed because they were first seen by us <6 months before the end of study period, and 9 had no DMSA study because of denied consent or loss to follow-up. An additional patient of the 95, who underwent a DMSA scan, was excluded because of cystinuria with staghorn calculi. This led to a final sample of 77 subjects without VUR studied (Table 1). At entry, 83 (40%) VUR and 29 (38%) no VUR patients under study had a temperature ≥39.5°C. The diagnosis of UTI preceding IC was made among the subjects aged <2 years by bladder catheterisation in 86 of 104 (83%) VUR and in 34 of 39 (87%) no VUR patients and by bag urine in the remaining ones, whereas among those aged >2 years the diagnosis was made on a midstream sample in 93 of 102 (91%) VUR and in 35 of 38 (92%) no VUR subjects and by bladder catheterisation in the remaining ones. In all, 137 VUR and 49 no VUR subjects had their first diagnosis of UTI when first seen by us.
Statistics
Student’s t-test, having verified the normal distribution of values, as well as the chi-square test and Fisher’s exact tests were used for statistical analysis. A p value of less than 0.05 was considered significant.
Results
A renal parenchymal defect was found in 83 of 206 (40%) VUR and in 5 of 77 (6%) no VUR subjects. The prevalences of subjects with any kind of renal defect, with focal defects of uptake, with split function <43%, as well as with most likely acquired defects were significantly higher among VUR than no VUR subjects (Table 2). Of the 83 VUR patients with renal defects, as much as 21 were <1 year old and 33 were <3 years old. In contrast, the five no VUR patients with renal defects were aged 6.5–10.3 years.
None of the no VUR and 12 of the VUR subjects had bilateral renal defects (p=0.067). All of the 12 VUR patients with bilateral renal defects (11 girls) had bilateral VUR; 10 of the 12 had bilateral focal defects of uptake and 7 of the 10 subjects with bilateral focal defects had a one kidney’s relative function <43%, whereas the remaining 2 had unilateral focal defects with the contralateral kidney split function <43%.
Renal defects were found in, respectively, 23 of 57 (40%) boys and in 60 of 149 (40%) girls with VUR (p=0.882) and in 1 of 25 (4%) boys and 4 of 52 (8%) girls without VUR (p=0.903). Among VUR patients, the estimated most likely acquired defects were found in 10 of 23 (43%) boys and in 45 of 60 (75%) girls (p=0.014).
Table 3 shows the prevalence of permanent renal defects according to grade of VUR, with the highest grade of reflux defining to which group each patient belongs. The prevalence of renal defects significantly increased with increase in the grade of reflux (Table 3). Among VUR subjects, renal defects were present in 91 of 306 (30%) of the refluxing and in 3 of 106 (3%) of the non-refluxing kidneys (p=0.0001).
All the patients under study had normal blood pressure. Three patients with VUR (two girls) and none without VUR had CCr below −2 SD of average normal values (62, 69 and 76 ml/min per 1.73 m2 at ages 2.2, 4 and 6.5 years, respectively).
Discussion
The prevalence of VUR in the present series (62%) is very high compared to most studies in the literature. We believe that this substantially depends on the higher sensitivity of IC that we used in detecting reflux. Actually, whereas part of the studies comparing X-ray VCU and IC reported rather similar rates of VUR detection with the two techniques [18–21] other reports [10–15] indicated enhanced detection of VUR with IC. The sample size, the selection of materials, as well as possible differences in the quality of exams [22] may explain the different findings. Unfortunately, in a number of reports, IC was performed only in patients with negative X-ray VCU, which makes a comparative analysis of the studies difficult. However, it is worth noting that the more recent and comprehensive reports [10, 14, 15], including a study by our group [10] in which both exams were performed in all patients with suspected reflux, indicated a higher sensitivity of IC in detecting VUR.
One may as well postulate that in the studies reporting a better performance of IC, the quality of X-ray VCU was inadequate, thus leading to underdiagnosis of reflux by the latter method. One way of estimating the adequacy of X-ray VCU in one report is to verify whether the rate of detected VUR corresponds to that expected on the basis of the age and of the clinical characteristics of the patients studied. In our quoted study comparing X-ray VCU and IC [10], VCU detected VUR in 28 of 124 (23%) of the children. The reported rates of refluxes detected with the classic VCU in subjects with antenatal hydronephrosis was estimated to be 24% in a review [2], but rates as low as 13 and 15% have been found in single comprehensive studies [23, 24]. Rates of 39% have been reported among infants and young children with febrile UTI [25]. Due to the trend of reflux to resolve with increasing age, a lower rate of VUR is expected among older children. Taking into account that in our quoted study [10] one-third of the patients was older than 3 years and that one-third was investigated for antenatal hydronephrosis, one can conclude that the rate of VUR detected by our X-ray VCU was not too dissimilar from that expected. Therefore, IC actually appears more sensitive in detecting VUR. This may depend on the continuous monitoring of bladder filling and voiding which enhances detection of transient reflux.
The inclusion of subjects investigated for antenatal hydronephrosis who have a lower rate of VUR than those with febrile UTI may explain the lower (41%) rate of VUR detected in our previous study by X-ray VCU and/or IC [10] than in the present series (62%).
The prevalence of persistent renal defects in the present series (40%) is much higher than that reported by Goldman et al. (19%) [26] and by Hoberman et al. (9.5%) [25] in infants and young children after their first UTI. The much higher age of our patients (3.2 years on average) with the possible cumulation of previous pyelonephritic episodes may explain the difference.
The main finding of the present study is that most—if not all—of the permanent renal defects was associated with VUR (Table 2). Actually, renal defects were detected in only 5 of 77 patients without VUR. All five patients were older than 6 years, an age when new renal scars seem less likely to develop [27]. In contrast, more than one-third of subjects with VUR and renal defects were under 3 years old. It is worth noting that if we had studied only infants and young children, as in many studies on the subject, we had found that permanent renal defects were always associated with VUR. It is tempting to hypothesize that, in the five older children without reflux and with renal defects, VUR could have spontaneously resolved before the UTI that prompted imaging studies. One cannot, however, dismiss the significantly (p=0.0001) higher prevalence of permanent renal defects in subjects with (40%) than in those without (6%) reflux. This is not surprising because many of the renal defects may be congenital due to an embryological problem associated with a ureteral bud abnormality and are unlikely in children without reflux. Acquired parenchymal defects can only be documented if a renal scintigraphy has been performed prior to and after a febrile UTI. In the present study, we performed only one scintigraphy 6 months after the UTI. However, we attempted to estimate which patients had most likely acquired defects by selecting those with focal uptake defects—suggesting renal damage acquired after UTI [6, 28]—and no severely reduced renal function. Interestingly, also the prevalence of subjects with most likely acquired defects was significantly (p=0.0001) higher in the group with (27%) than without (5%) reflux. It is known that, among children with VUR, the majority of subjects with congenital defects are boys whereas the majority of those with acquired post-infectious defects are girls [28]. Accordingly, in the present series, girls were significantly (p=0.014) more prevalent than boys among the subset with reflux and most likely acquired defects.
Most renal defects may have little or no clinical significance for the patients’ future health, especially when only one kidney is affected. However, in the present study, a split function below 43%, which may be considered an index of severity of the renal defect, was significantly (p=0.0001) more prevalent in VUR (26%) than in no VUR (5%) subjects. Moreover, renal defects were bilateral and hence potentially threatening to long-term global renal function in 12 VUR patients and in none without VUR, although the difference did not reach significance (p=0.067). Finally, a CCr below −2 SD of average normal values, possibly heralding a further decline of renal function, was recorded in three VUR patients and in none without VUR. Chronic renal insufficiency or failure has been associated with primary VUR [1, 2, 29, 30], although it may be difficult to disentangle the role of a VUR-associated congenital renal defect from that of a renal defect secondary to UTI. In contrast, to our knowledge, a clear association of pyelonephritic damage in the absence of reflux with renal insufficiency or failure has not been reported in the literature. This further supports the role of VUR in permanent renal defects.
The role of VUR in the genesis of renal defects has been questioned due to the finding of renal defects in many children with UTI and no demonstrable reflux [7]. It is paradoxical, however, that all the studies on the subject [25, 26, 31], including the present one (Tables 2 and 3), found a strict association between the presence and the grade of VUR and the occurrence of renal defects. On the other hand, it has recently been reported that children with higher grade reflux and abnormal DMSA scan are at increased risk of breakthrough UTI [31]. The occurrence of an undetected reflux in most instances of renal defects with no demonstrable VUR, which represents the main suggestion of the present report, could explain that paradox. Intermittent or “occult” VUR is a well-established phenomenon and its occurrence has recently been stressed by Rubenstein et al. [32]. They, by positioning the instillation of contrast at the ureteral orifice at the time of cystoscopy, showed VUR in none of the controls and in all of the 30 children in their study who experienced febrile UTI and yet did not have reflux on standard cystography.
Our findings seem in contrast with the meta-analysis by Gordon et al. [7] in which VUR was found to be a weak predictor of renal defects in children hospitalized with febrile UTI. It should be noted that most DMSA scans in the studies reviewed in the meta-analysis were done within 6 weeks of the UTI and that VUR was detected by the classic X-ray VCU. The fact that the comparable prevalences of renal defects among subjects with and without VUR included acute pyelonephritic damage as well as the possible occurrence of a reflux overlooked by the classic VCU may explain the different findings with respect to our study.
Our diagnostic protocol included a late DMSA renal scanning in all children with VUR and only in those of the no VUR subjects with at least two febrile UTI or with therapeutic delay. Nevertheless, this selection bias does not lessen, but strengthens, our main conclusion that most permanent renal defects in children with febrile UTI are associated with VUR, because only those no VUR subjects who were considered to be at higher risk for renal defects were selected for DMSA study.
Overall, in our study, permanent renal defects after febrile UTI were much more frequent and severe in children with reflux. This suggests that acute renal damage is less severe or/and that it may heal more easily in the absence of VUR.
The infrequent occurrence of renal defects in children with febrile UTI and no VUR challenges the use of antibacterial prophylaxis in these patients which is recommended by some experts, and the practice of doing DMSA scans in the absence of reflux. However, definite advice on this matter can only be given after reliable prospective randomised studies have been performed. Our findings also indicate that the possibility that a child will have permanent renal defects can reasonably be ruled out on the basis of the absence of VUR at IC. Bladder catheterisation is an invasive and unpleasant procedure. Nevertheless, IC implies less radiation exposure than a DMSA scan. Moreover, IC identifies the population of subjects with UTI which is at risk for permanent renal defects and which will require surveillance and possible therapeutic measures. Therefore, we propose doing IC as the first-line investigation in infants and children with febrile UTI and searching for permanent renal defects in those with VUR by late DMSA scan.
References
Lin DS, Huang SH, Lin CC, Tung YC, Huang TT, Chiu NC, Koa HA, Hung HY, Hsu CH, Hsieh WS, Yang DI, Huang FY (2000) Urinary tract infection in febrile infants younger than eight weeks of age. Pediatrics 105:E20
Jacobson SH, Hansson S, Jakobsson B (1999) Vesicoureteric reflux: occurrence and long-term risks. Acta Paediatr Suppl 43:22–30
Patzer L, Seeman T, Luck C, Wuhl E, Janda J, Misselwitz J (2003) Day-and night-time blood pressure elevation in children with higher grades of renal scarring. J Pediatr 142:117–122
McGladdery SJ, Aparicio S, Verrier-Jones K, Roberts R, Sacks SH (1992) Outcome of pregnancy in an Oxford-Cardiff cohort of women with previous bacteriuria. Q J Med 83:533–539
Goldraich NP, Ramos AL, Goldraich IH (1989) Urography versus DMSA scan in children with vesicoureteric reflux. Pediatr Nephrol 3:1–5
Jakobsson B, Svensson L (1997) Transient pyelonephritic changes on 99mTechnetium-dimercaptosuccinic acid scan for at least 5 months after infection. Acta Paediatr 86:803–807
Gordon I, Barkovics M, Pindoria S, Cole TJ, Woolf AS (2003) Primary vesicoureteric reflux as a predictor of renal damage in children hospitalized with urinary tract infection: a systematic review and meta-analysis. J Am Soc Nephrol 14:739–744
Paltiel HJ, Rupic RC, Kiruluta HG (1992) Enhanced detection of vesicoureteral reflux in infants and children with use of cyclic voiding cystourethrography. Radiology 184:753–755
Polito C, Moggio G, La Manna A, Cioce F, Cappabianca S, Di Toro R (2000) Cyclic voiding cystourethrography in the diagnosis of occult vesicoureteric reflux in infants and children. Pediatr Nephrol 14:39–41
Polito C, Rambaldi PF, La Manna A, Mansi PL, Di Toro R (2000) Enhanced detection of vesicoureteric reflux with isotopic cystography. Pediatr Nephrol 14:827–830
Nasrallah PF, Nara S, Crawford J (1982) Clinical applications of nuclear cystography. J Urol 128:550–553
Fretzaias A, Karpathios T, Dimitriou P, Nicolaidou P, Matsaniotis N (1984) Grading of vesicoureteral reflux by radionuclide cystography. Pediatr Radiol 14:148–150
Kogan SJ, Sigler L, Levitt SB, Reda EF, Weiss R, Greifer I (1986) Elusive vesicoureteral reflux in children with normal contrast cystograms. J Urol 136:325–328
Saraga M, Stanicic A, Markovic V (1996) The role of direct radionuclide cystography in evaluation of vesicoureteral reflux. Scand J Urol Nephrol 30:367–371
Poli-Merol ML, Francois S, Pfliger F, Lefebvre F, Roussel B, Liehn JC, Daoud S (1998) Interest of direct radionuclide cystography in repeated urinary tract infection exploration in childhood. Eur J Pediatr Surg 8:339–342
Schwartz GJ, Brion LP, Spitzer A (1987) The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children and adolescents. Pediatr Clin North Am 34:571–590
National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents (1996) Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: a working group report from the National High Blood Pressure Education Program. Pediatrics 98:649–658
Brendstrup L, Carlsen N, Nielsen SL, Dyrbye M, Eiken M, Krasilnikoff PA, Gertz TC (1983) Micturition cystourethrography using X-ray or scintigraphy in children with reflux. Acta Paediatr Scand 72:559–562
Dikshit MP, Acharya VN, Shikare S, Merchant S, Pardanani DS (1993) Comparison of direct radionuclide cystography with micturating cystourethrography for the diagnosis of vesicoureteric reflux and its correlation with cystoscopic appearances of the ureteric orifices. Nephrol Dial Transplant 8:600–602
Zechmann W, Raushmeier H, Dumfahrt H, Fill H, Joost J, Riccabona G (1983) Value and indication for isotope reflux cystography in children. Acta Medica Austriaca 10:57–60
Zhang G, Day DL, Loken M, Gonzalez R (1987) Grading of reflux by radionuclide cystography. Clin Nucl Med 12:106–109
Tamminen-Mobius T, Brunier E, Ebel KD, Lebowitz R, Olbing H, Seppanen U, Sixt R (1992) Cessation of vesicoureteral reflux for 5 years in infants and children allocated to medical treatment. The International Reflux Study in Children. J Urol 148:1662–1666
Anderson NG, Abbott GD, Mogridge N, Allen RB, Maling TM, Wells JE (1997) Vesicoureteric reflux in the newborn: relationship to fetal pelvic diameter. Pediatr Nephrol 11:610–616
Phan V, Traubici J, Hershenfield B, Stephens D, Rosenblum ND, Geary DF (2004) Vesicoureteral reflux in infants with isolated antenatal hydronephrosis. Pediatr Nephrol 19:819–820
Hoberman A, Charron M, Hickey RW, Baskin M, Kearney DH, Wald ER (2003) Imaging studies after a first febrile urinary tract infection in young children. N Engl J Med 348:195–202
Goldman M, Bistritzer T, Horne T, Zoareft I, Aladjem M (2000) The etiology of renal scars in infants with pyelonephritis and vesicoureteral reflux. Pediatr Nephrol 14:385–388
Olbing H, Smellie JM, Jodal U, Lax H (2003) New renal scars in children with severe VUR: a 10-year study of randomised treatment. Pediatr Nephrol 18:1128–1131
Wennerstrom M, Hansson S, Jodal U, Stokland E (2000) Primary and acquired renal scarring in boys and girls with urinary tract infection. J Pediatr 136:30–34
Ardissino G, Dacco V, Testa S, Bonaudo R, Claris-Appiani A, Taioli E, Marra G, Edefonti A, Sereni F, ItalKid Project (2003) Epidemiology of chronic renal failure in children: data from the ItalKid project. Pediatrics 111:e382–e387
Smellie JM, Barratt TM, Chantler C, Gordon I, Prescod NP, Ransley PG, Woolf AS (2001) Medical versus surgical treatment in children with severe bilateral vesicoureteric reflux and bilateral nephropathy: a randomized trial. Lancet 357:1329–1333
Mingin GC, Nguien HT, Baskin LS (2004) Abnormal dimercapto-succinic acid scans predict an increased risk of breakthrough infection in children with vesicoureteral reflux. J Urol 172:1075–1077
Rubenstein JN, Maizels M, Kim S, Houston JTB (2003) The PIC cystogram: a novel approach to identify “occult” vesicoureteric reflux in children with febrile urinary tract infections. J Urol 169:2339–2343
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Polito, C., Rambaldi, P.F., Signoriello, G. et al. Permanent renal parenchymal defects after febrile UTI are closely associated with vesicoureteric reflux. Pediatr Nephrol 21, 521–526 (2006). https://doi.org/10.1007/s00467-006-0036-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-006-0036-3