Abstract
There have been few reports on immune complex-mediated glomerulonephritis associated with chronic infection from long-term central venous catheterization in adulthood. We report here on a 13-year-old boy with nephritis who exhibited glomerulonephritis that had been induced by the long-term use of central venous catheters, and its resolution after extraction of the central venous catheter. A diagnosis of glomerulonephritis associated with chronic infection caused by long-term central venous catheterization was made, based on the absence of clinical findings after removal of the catheter, hypocomplementemia, pathology findings resembling membranoproliferative glomerulonephritis, and detection of Staphylococcus epidermidis from culture of the removed catheter culture. For clinicians using long-term central venous access for parenteral feeding, rapid catheter exchange is necessary for patients with fever of unknown origin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Home parental nutrition has become the standard of care for patients unable to maintain adequate nutrition through the gastrointestinal tract, including patients with conditions such as short bowel syndrome, chronic intestinal pseudo-obstruction, bowel ischemia, radiation enteritis, and high-output entero-cutaneous fistulas.
There have been many reports of complications in patients undergoing home parenteral nutrition, including infection, venous thrombosis, and catheter migration [1]. However, there have been few reports on immune complex-mediated glomerulonephritis associated with chronic infection from long-term central venous catheterization [2].
We report here on a 13-year-old boy with nephritis who exhibited pathology findings such as membranoproliferative glomerulonephritis (MPGN) that had been induced by such a catheter, and its resolution after extraction of the catheter.
Case report
A 13-year-old boy had received home parenteral nutrition since he had been 1 month of age for short bowel syndrome secondary to midgut volvulus and resection of necrotic ileum. The patient had been having episodes of sepsis induced by central vein catheterization since 1 year of age. His catheter had been changed a total of seven times.
From January 2003 he had had a high fever once a month, but the clinical findings disappeared following administration of antibiotics. In May 2004 hematuria and proteinuria were detected at school urinary screening. He was referred to our hospital and admitted for examination.
On admission, his blood pressure was 110/64 mmHg and he had no purpura and no edema. The chest and abdomen exhibited no abnormal finding except operation scars. The catheter exit site was without erythema, and there was no tenderness around the tunneled portion of the catheter.
The results of laboratory tests revealed a leukocyte count of 9,200/mm3; erythrocyte count of 449×104/mm3; platelet count of 19.0×104/mm3; serum total protein of 7.0 g/dl; serum albumin of 3.7 g/dl; serum creatinine of 0.6 mg/dl; and total cholesterol of 296 mg/dl. Urinalysis revealed protein 0.3 g/day; β-2MG 274μg/ml; NAG 14 U/l; and sediment containing many erythrocytes per high-power field, 10 to 15 leukocytes, and 1 to 2 granular casts per high-power field. His 24 h creatinine clearance (24-h Ccr) was 84.6 ml/min per 1.73 m2 body surface area. Immunological studies revealed: third component of complement (C3, normal range 69–128 mg/dl) of 30 mg/dl; C4 (14–36 mg/dl) of 8 mg/dl; CH50 (30–45 U/ml) <10 U/ml; anti-nuclear antibody titer of 80; anti-DNA antibody 0 IU/ml; IgG 1,530 mg/dl (normal range 1,188–1,800 mg/dl); IgA 408 mg/dl (normal 158–358 mg/dl); IgM 120 mg/dl (normal 72–216 mg/dl); titer of antibody to hepatitis B virus, negative; titer of antibody to hepatitis C virus, negative; and titer of antibody to syphilis, negative.
After admission he had continuous fever and findings of an inflammatory response. The cause of continuous fever was unknown. He was treated with the antibiotic ceftazidime and panipenem-betamipron. Because of suspicion of infection from the central venous catheter, the catheter was removed. The fever remitted following removal of the catheter. The removed catheter and blood cultures grew Staphylococcus epidermidis.
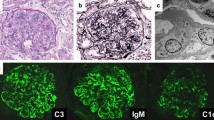
A percutaneous renal needle biopsy was performed after he had been in hospital for 14 days. On immunofluorescence microscopy, C1, C3, and IgM deposits with fringe pattern were found. Light microscopy (LM) with periodic acid–Schiff (PAS) stain revealed mesangial proliferation, increased lobulation in the mesangium, and thickening of capillary walls; LM with periodic acid–methenamine (PAM) stain revealed double contours (Fig. 1).
After catheter removal, the proteinuria, hematuria, and hypocomplementemia disappeared. Therefore, a diagnosis of glomerulonephritis associated with chronic infection from long-term central venous catheterization was made, based on the absence of clinical findings after catheter removal, hypocomplementemia, pathology findings, including MPGN, and detection of Staphylococcus epidermidis from culture of the removed catheter. The boy has had no urinary abnormality for 1 year.
Discussion
The development of tunneled central venous catheters that are durable and easy to care for has tremendously improved the quality of life of patients who cannot maintain adequate nutrition through the gastrointestinal tract. Home parenteral nutrition has become the standard of care for these patients. Tunneled central venous catheters have multiple applications, including administration of antibiotics, chemotherapy, and fluids. These catheters can cause several complications, including infection, venous thrombosis, and catheter migration, with possible embolization [3]. Infection, one of the most common complications from these catheters, usually presents with fever. However, there have been a few reports of immune complex-mediated glomerulonephritis associated with chronic infection caused by the long-term use of central venous catheters. Yared et al. reported on two patients who had developed MPGN secondary to occult infection from a tunneled right atrial catheter used for home parenteral nutrition [2].
In our patient we diagnosed glomerulonephritis associated with chronic infection caused by long-term central venous catheterization, based on the absence of clinical findings after removal of the catheter, the hypocomplementemia, pathology findings, including MPGN, and detection of Staphylococcus epidermidis from culture of the removed catheter. These serological and immunofluorescence findings indicated activation of the classical complement pathway.
MPGN is characterized morphologically by endocapillary proliferation, increased mesangial matrix and duplication, and/or thickening of the glomerular basement membrane, and may be a primary glomerular disease or secondary to a variety of infections or hereditary or multi-system diseases.
We speculated that the pathogenesis of glomerulonephritis resembling MPGN and associated with chronic infection from long-term central catheterization was as follows [4, 5, 6, 7]:
-
1.
Circulating immune complexes containing IgA and IgG in the circulation are formed in response to Staphylococcus epidermidis infection.
-
2.
Deposition of these circulating immune complexes in the kidney result in development of glomerulonephritis resembling MPGN.
-
3.
Several cytokines induced by circulating immune complexes and sepsis may play important roles in the pathogenesis of glomerulonephritis resembling MPGN.
In conclusion, we have reported here on a 13-year-old boy with glomerulonephritis associated with chronic infection from the long-term use of central venous catheters. For clinicians using long-term central venous access for parenteral feeding, rapid catheter exchange is necessary for patients with fever of unknown origin.
References
Black JA, Chaacombe DN, Ockenden BG (1965) Nephrotic syndrome associated with bacteraemia after shunt operations for hydrocephalus. Lancet 2:921–924
Yared G, Seidner DL, Steiger E, Hall PM, Nally JV (1999) Tunneled right atrial catheter infection presenting as renal failure. JPEN J Parenter Enteral Nutr 23:363–365
Groeger JS, Lucas AB, Thaler HT (1993) Infectious morbidity associated with long-term use of venous access devices in patients with cancer. Ann Intern Med 119:1168–1174
Wyatt RJ, Walsh JW, Holland NH (1981) Shunt nephritis: the role of the complement system in its pathogenesis and management. J Neurosurg 55:99–107
Schoeneman M, Bennet B, Greifer B, Greifer I (1982) Shunt nephritis progressing to chronic renal failure. Am J Kidney Dis 2:375–377
Arze RS, Rashid H, Morley R, Ward MK, Kerr DNS (1983) Shunt nephritis. Report of two cases and review of the literature. Clin Nephrol 19:48–53
Haffner D, Schindera F, Aschoff A, Matthias S, Waldherr R, Scharer K (1997) The clinical spectrum of shunt nephritis. Nephrol Dial Transpl 12:1143–1148
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ohara, S., Kawasaki, Y., Takano, K. et al. Glomerulonephritis associated with chronic infection from long-term central venous catheterization. Pediatr Nephrol 21, 427–429 (2006). https://doi.org/10.1007/s00467-005-2124-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-005-2124-1