Abstract
To identify the factors affecting histological regression of crescentic Henoch–Schönlein nephritis (HSN), we retrospectively analyzed serially biopsied 20 children with crescentic HSN treated with immunosuppressants. They were classified into two groups according to the histological changes between the first and second biopsy: group I (n=10) with histological regression and group II (n=10) with no change or histological progression. Of the 20 patients, 19 showed a favorable outcome at the end of follow-up. Initial laboratory and histological findings did not differ between the two groups. Histological regression was associated with a younger age at onset (P=0.003), early treatment with immunosuppressants (P=0.044) and absent or decreased fibrinogen deposits at the second biopsy (P<0.0001) in a univariate analysis. Mesangial IgA and fibrinogen depositions at the second biopsy were reduced significantly in group I (P<0.05). In the multivariate analysis, a younger age was an independent determinant of histological regression (OR 1.44; 95% CI 1.03–2.02). The intensity of fibrinogen deposits at the second biopsy correlated positively with the age at onset (r=0.503, P=0.024), and the chronicity index at the second biopsy correlated positively with the time that immunosuppressive therapy was started (r=0.619, P=0.004).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although the prognosis for unselected children with Henoch–Schönlein purpura (HSP) is relatively good, severe nephritis remains the major cause of morbidity and mortality in patients with HSP [1, 2, 3, 4]. Especially, the risk for developing chronic renal insufficiency is highest in children with crescents in more than half of the glomeruli [5].
Some studies reported a beneficial effect of several agents, such as intravenous methylprednisolone pulse, azathioprine, cyclophosphamide, anticoagulants, and urokinase, in treating severe Henoch–Schönlein nephritis (HSN), using follow-up biopsy [6, 7, 8, 9, 10, 11, 12, 13], but routine biopsy for patients who have recovered or are in stable remission without treatment is not currently recommended.
However, long-term studies assessing the prognosis of HSN have shown that clinical recovery does not inevitably mean favorable long-term outcome [1, 2]. According to Goldstein et al., of 78 patients with a mean follow-up of 23.4 years after onset, 17 had clinically deteriorated, and, of these 17, seven had completely recovered after a follow-up period of 10 years [1].
Therefore, it is important to induce histological regression in treating severe HSN and to identify the factors associated with histological regression or progression.
In this study, we retrospectively compared children with HSN who showed regression of the International Study of Kidney Disease in Children (ISKDC) grade in a follow-up biopsy with those who did not, with respect to their clinical characteristics and several histological disease markers in the initial and the follow-up biopsy, including activity and chronicity index, tubulointerstitial score and immunoglobulin staining.
Methods
Patients
We retrospectively analyzed serially biopsied 20 children with crescentic HSN [at least ISKDC grade IIIa, focal proliferation with<50% crescents; see Histopathology section] from 1990 to 2002 (mean age 9.2 years, range 3.7–13.8 years). There were 14 boys and 6 girls. The mean duration of follow-up was 5.8 years (range 1–12 years). HSN was diagnosed when hematuria and/or proteinuria were associated with a characteristic purpuric eruption and/or abdominal or joint pain (at least two of these three clinical signs). All patients were treated with steroids and immunosuppressants (azathioprine or cyclosporine) due to severe renal symptoms such as nephritic syndrome, nephrotic syndrome, or both, proteinuria (>1 g/day) and biopsy specimen grade III or more. The dose of azathioprine was 2 mg/kg per day, and it was given for 8 months. The starting dose of cyclosporine was 5 mg/kg per day and the desired drug level was kept at 100–200 ng/ml (trough levels). The duration of cyclosporine therapy ranged from 8 months to 12 months and did not differ between the two groups. They were classified into two groups according to the histological changes between the first and second biopsy: group I (n=10) with improvement in the ISKDC grade; and group II (n=10) with no change or deterioration in the ISKDC grade. This study was approved by the institutional review board and the research ethics committee of Yonsei Severance Hospital.
Evaluation of clinical status
The clinical status of each patient at the end of therapy and at the latest follow-up was classified as follows [14]:
-
State A. Normal: normal physical examination results, urine, and renal function.
-
State B. Minor urinary abnormalities: normal on physical examination with microscopic hematuria or proteinuria less than 40 mg/m2 per hour
-
State C. Active renal disease: proteinuria of 40 mg/m2 per hour, or greater, or hypertension and glomerular filtration rate (GFR) of 60 ml/min per 1.73 m2 or greater
-
State D. Renal insufficiency: GFR less than 60 ml/min per 1.73 m2 (including dialysis/transplantation or death)
Histopathology
A renal biopsy was obtained in all patients before and after therapy. The interval between the first and second biopsy was 8 to 12 months (mean 10.8 months). The glomerular changes were graded according to the classification of the ISKDC) (grade I minimal alterations; grade II mesangial proliferation; grade IIIa focal, IIIb diffuse proliferation with<50% crescents; grade IVa focal, IVb diffuse proliferation with 50–75% crescents; grade Va focal, Vb diffuse proliferation with >75% crescents; grade VI membranoproliferative glomerulonephritis) [15]. Renal biopsy specimens were also scored semiquantitatively with the scoring system of Andreoli and Bergstein for IgA nephropathy [8] modified by Foster et al. [16]. Acute changes included mesangial hypercellularity (0–3), mesangial matrix increase (0–3), endothelial swelling (0–2), interstitial mononuclear infiltrate (0–2), interstitial edema (0–2), tubular damage (0–2), cellular crescents (0–3), basement membrane adhesion to Bowman’s capsule (0–3), glomerular neutrophils (0–3), and fibrinoid necrosis (0–3). Chronic renal injury was estimated by interstitial fibrosis and tubular atrophy (0–2), fibrous crescents (0–3), global sclerosis (0–3), and vascular hyalinosis and intimal hyperplasia (0–1). The sum of these numbers comprised the activity index and the chronicity index, respectively. The scores from four factors (interstitial mononuclear infiltrate, interstitial edema, tubular damage, and interstitial fibrosis and tubular atrophy) were combined into the tubulointerstitial (TI) scores. The percentage of glomeruli with crescents (epithelial or fibrous) was also expressed [17]. For the microscopic immunofluorescence (IF) examination a portion of the fresh renal tissue was frozen and exposed to fluorescein isothiocyanate (FITC)-conjugated antihuman IgG, IgA, IgM, C3, and fibrinogen. The overall intensity of immunofluorescence was also scored semiquantitatively where 0 = negative, 0.5 = trace, 1 = mild, 2 = moderate, and 3 = severe.
Statistical methods
Statistics were done with SPSS (version 11.0 for Windows). Continuous variables were expressed as mean values ± standard error of the mean. Variables were compared by Mann–Whitney test, Fisher’s exact test, and Wilcoxon signed-rank test. The stepwise logistic regression analysis was used for a multivariate analysis. We changed some continuous variables (age, the semiquantitative scoring of fibrinogen deposits) to categorical variables (age >9 years or not, and the absence/decrease or persistence/increase of fibrinogen deposits at the second biopsy) to perform logistic regression analysis. Correlation between two variables was assessed by Spearman’s rank-correlation test. All differences were considered significant at P<0.05.
Results
Clinical characteristics and laboratory findings
There were no differences between the two groups in gender, hypertension, initial renal manifestations, and treatment. However, the patients in group I were significantly younger than those in group II (7.4±1.1 years vs 11.0±0.8 years, P=0.019). The interval between the onset of disease and the start of therapy was significantly shorter in group I than in group II (3.5±1.2 weeks vs 7.6±1.5 weeks, P=0.044). The mean duration of follow-up was 5.2 years in group I and 6.4 years in group II and did not differ between the two groups. Although one patient in group II progressed to chronic renal insufficiency, there were no significant differences between the two groups in their clinical state at the end of therapy and at the latest follow-up (Table 1).
There were no significant differences between the two groups in urinary protein excretion, serum albumin, creatinine, and creatinine clearance (CCr) at the first biopsy. However, urinary protein excretion at the second biopsy was different between the two groups (0.1±0.03 g/m2 per day in group I vs 0.6±0.1 g/m2 per day in group II, P=0.004). In both groups, urinary protein excretion decreased significantly and serum albumin increased after therapy, whereas serum creatinine and CCr did not change (Table 2).
Histological findings
The mean interval between the first and second biopsy did not differ between the two groups (10.6 months in group I vs 11.2 months in group II). Light microscopy findings of the patients divided into two groups according to the study protocol are shown in Table 3. At the first biopsy, activity indices and the percentage of crescents did not differ between the two groups. The second biopsy of patients in group I showed a decrease in the activity index and the percentage of crescents, whereas the second biopsy of patients in group II showed a persistent increase in the activity index and the percentage of crescents. There were no differences between the two groups with regard to the chronicity index and TI scores of the first renal biopsy. However, the second renal biopsy revealed that the increase in the chronicity index and TI scores was significant only in group II. The changes in these scores were not significant in group I.
IF findings of the 20 patients are shown in Table 4. At the first and second biopsy, degree of deposits of immunoglobulins such as IgG, IgA, IgM, and C3 did not differ between the two groups. Intraglomerular fibrinogen deposition at the first biopsy was also not different. At the second biopsy, however, degree of fibrinogen deposits was more intense in group II than in group I (P=0.026). In group I, absent or decreased fibrinogen deposits at the second biopsy were observed in eight of the ten patients but were observed in none of the ten patients in group II (P<0.0001). Mesangial IgA deposition at the second biopsy was reduced significantly in group I (P=0.021).
Predictive factors for histological regression in crescentic HSN
A univariate analysis revealed that histological regression was related to an age of less than 9 years (8 vs 1, P=0.003), early treatment with immunosuppressants (3.5±1.2 weeks vs 7.6±1.5 weeks, P=0.044), and absent or decreased fibrinogen deposition at the second biopsy (8 vs 0, P<0.0001). In the multivariate analysis, a younger age was an independent predictive factor of histological regression (P=0.034; odds ratio 1.44; 95% confidence interval 1.03–2.02). Early treatment with immunosuppressants (P=0.096; odds ratio 1.31; 95% confidence interval 0.95–1.79) and fibrinogen deposition at the second biopsy (P=0.201; odds ratio 12.1; 95% confidence interval 0.26-551.5) were not.
Prediction of post-therapeutic activity index, chronicity index, and intraglomerular fibrinogen deposits by correlations among variables
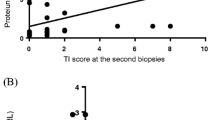
The activity index at the second biopsy correlated positively with changes in mesangial IgA deposits (post-therapy IgA deposits−pre-therapy IgA deposits, ΔIgA deposits) (activity index after therapy = 5.394+1.119×ΔIgA deposits, r=0.448, P=0.047) (Fig. 1a). The intensity of fibrinogen deposits at the second biopsy correlated positively with the age at onset [fibrinogen deposits after therapy = 0.084+0.08 × age at onset (years), r=0.503, P=0.024] (Fig. 1b). The chronicity index at the second biopsy correlated positively with the time immunosuppressive therapy was started (chronicity index after therapy = 0.383+0.156 × interval between the onset of disease and the start of therapy (weeks), r=0.619, P=0.004) (Fig. 1c).
a Correlation of the activity index at the second biopsy with changes in mesangial IgA deposits. b Correlation of intraglomerular fibrinogen deposits at the second biopsy with the age at onset. c Correlation of the chronicity index at the second biopsy with the time immunosuppressive therapy was started
Discussion
There have been few reports describing histological effects of immunosuppressive drugs on severe HSN using follow-up biopsy [6, 7, 8, 9, 10, 11, 12, 13]. Long-term studies assessing the prognosis of HSN have shown that clinical recovery does not always mean favorable long-term outcome [1, 2]. Goldstein et al. studied former HSP patients 23.4 years later and reported highly unpredictable outcomes, in that several patients with mild initial disease and apparent complete recovery showed chronic renal insufficiency after two decades [1]. Although the authors could not find any plausible explanation, Niaudet and Habib suggested that these results might be due to scarring of the renal parenchyma as a sequella of previous extensive glomerular damage during the acute episode, with a risk of progression [6]. Ronkainen et al. also reported that even patients with mild renal symptoms at the onset of HSP do carry a risk for severe long-term complications [2]. In addition, Algoet and Proesmans performed a follow-up renal biopsy 2–9 years (median 5.5 years) after the HSP episode and found that renal histology was normal in only one of the four patients who had achieved complete clinical remission 5–9 years after the onset of the HSP [18].
We hypothesized that even patients with clinical remission might have different histological changes after therapy, and focused on the histological heterogeneity after immunosuppressive therapy. Our results showed that renal histological findings after therapy were abnormal in all patients, regardless of histological regression, suggesting that the kidneys were not completely healed even in patients with clinical remission.
In our study, 19 of the 20 patients with crescentic HSN had a favorable outcome at the end of follow-up, but histological regression was achieved in only ten patients, confirming that clinical remission does not always mean histological improvement, and the histological reversibility of crescentic HSN is much less than clinical improvement. On this point, we studied the predictive factors for histological regression, and an age of less than 9 years, early treatment with immunosuppressants, and absent or decreased fibrinogen deposition at the second biopsy were found to be significant predictive factors of histological regression.
Although several studies have suggested a correlation between clinical outcome and age at onset, with children older than 5–10 years of age having a worse prognosis [5], there has been no study as to the effect of age on histological changes after immunosuppressive therapy in crescentic HSN. Our observations showed the age at HSP onset affects histological alterations after immunosuppressive therapy, with patients younger than 9 years of age showing a decrease in the activity index without an increase in the chronicity index and TI scores. Although previous studies demonstrating therapeutic benefit in HSN have not described loss of mesangial IgA deposits [7, 8, 9], our study showed that the post-therapeutic activity index had decreased with decreasing intensity of mesangial IgA deposits, suggesting mesangial IgA deposition might have an important role in the progression of immunologic renal injury and histopathologic changes in crescentic HSN.
Tanaka et al. have shown the importance and efficacy of early treatment of severe proteinuric HSN [9], and all nine patients with nephrotic-range proteinuria treated with prednisolone and oral cyclophosphamide within a month of HSP diagnosis showed improvement in symptoms and histological findings (From ISKDC IIIb or IVb to II). Our results showed that early treatment with immunosuppressants would be an important factor to achieve histological regression by decreasing chronic renal injury. The interval between the onset of HSP and the start of therapy varied, because some patients in group II were referred to our center from a secondary hospital, which might have delayed treatment and influenced the outcome, although the first biopsy findings and initial renal symptoms did not differ between the two groups.
It has been well known that intraglomerular fibrinogen deposition plays an important role in the pathogenesis of experimental crescentic glomerulonephritis as a mediator of injury [19, 20, 21]. Although less extensively studied in humans [22], the beneficial effects of urokinase in Japanese patients with severe HSN also indicate an important functional role for fibrinogen [10, 23]. We also showed that the number of glomerular crescents was reduced according to the disappearance of fibrinogen deposition, and post-therapeutic fibrinogen deposition was influenced by the age at onset.
Although some authors had recommended follow-up biopsy in only patients with persistent severe renal symptoms, such as heavy proteinuria [24], we demonstrated the utility of follow-up biopsy in crescentic HSN. Firstly, initial biopsy findings could not predict histological regression, but decreased mesangial IgA or fibrinogen deposition at follow-up biopsy was an important predictive factor for histological regression. Secondly, we could identify chronic and tubulointerstitial changes with follow-up biopsy, which are known to be more predictive of long-term outcome than are glomerular changes [25]. Although cyclosporine was used in 12 patients (seven in group I and five in group II), the characteristic lesion of cyclosporine-induced nephrotoxicity at the second biopsy was not found in both groups. Nevertheless, a recommendation for a follow-up biopsy after therapy might only be justified in the case of a proven clinical significance of the biopsy result, because the importance of the histological regression for the patient outcome has not been shown.
There are some limitations in this study, such as the small sample size, short follow-up period, and the retrospective nature of the study. Also, we could not find any association between histological regression of this disease and a favorable long-term outcome. Nevertheless, the present study at least showed the discrepancy between clinical improvement and histological regression in immunosuppressant-treated children with crescentic HSN, suggesting the heterogeneous nature of this disease.
In conclusion, younger patients who have been treated earlier may have more favorable post-therapeutic histological findings, and the decrease of fibrinogen deposits in immunofluorescence examination can predict the histological regression in children with crescentic HSN.
References
Goldstein AR, White RHR, Akuse R, Chantler C (1992) Long-term follow-up of childhood Henoch–Schönlein nephritis. Lancet 339:280–282
Ronkainen J, Nuutinen M, Koskimies O (2002) The adult kidney 24 years after childhood Henoch–Schönlein purpura. A retrospective cohort study. Lancet 360:666–670
Chang WL, Yang YH, Wang LC, Lin YT, Chiang BL (2005) Renal manifestations in Henoch–Schönlein purpura: a 10-year clinical study. Pediatr Nephrol 20:1269–1272
Halling SF, Soderberg MP, Berg UB (2005) Henoch–Schönlein nephritis: clinical findings related to renal function and morphology. Pediatr Nephrol 20:46–51
Rai A, Nast C, Adler S (1999) Henoch–Schönlein purpura nephritis. J Am Soc Nephrol 10:2637–2644
Niaudet P, Habib R (1998) Methylprednisolone pulse therapy in the treatment of severe forms of Schönlein–Henoch nephritis. Pediatr Nephrol 12:238–243
Iijima K, Ito-Kariya S, Nakamura H, Yoshikawa N (1998) Multiple combined therapy for severe Henoch–Schönlein nephritis in children. Pediatr Nephrol 12:244–248
Foster BJ, Bernard C, Drummond KN, Sharma AK (2000) Effective therapy for severe Henoch–Schönlein purpura nephritis with prednisone and azathioprine: a clinical and histopathologic study. J Pediatr 136:370–375
Tanaka H, Suzuki K, Nakahata T, Ito E, Waga S (2003) Early treatment with oral immunosuppressants in severe proteinuric purpura nephritis. Pediatr Nephrol 18:347–350
Kawasaki Y, Suzuki J, Nozawa R, Suzuki S, Suzuki H (2003) Efficacy of methylprednisolone and urokinase pulse therapy for severe Henoch–Schönlein nephritis. Pediatrics 111:785–789
Tarshish P, Bernstein J, Edelmann CM Jr (2004) Henoch–Schönlein purpura nephritis: course of disease and efficacy of cyclophosphamide. Pediatr Nephrol 19:51–56
Kawasaki Y, Suzuki J, Murai M, Takahashi A, Isome M, Nozawa R, Suzuki S, Suzuki H (2004) Plasmapheresis therapy for rapidly progressive Henoch–Schönlein nephritis. Pediatr Nephrol 19:920–923
Shin JI, Park JM, Shin YH, Kim JH, Kim PK, Lee JS, Jeong HJ (2005) Cyclosporin A therapy for severe Henoch–Schönlein nephritis with nephrotic syndrome. Pediatr Nephrol 20:1093–1097
Meadow SR, Glasgow EF, White RH, Moncrief MW, Cameron JS, Ogg CS (1972) Schönlein–Henoch nephritis. Q J Med 41:241–258
Counahan R, Winterborn MH, White RH, Heaton JM, Meadow SR, Bluett NH, Swetschin H, Cameron JS, Chantler C (1977) Prognosis of Henoch–Schönlein nephritis in children. BMJ 2:11–14
Andreoli SP, Bergstein J (1989) Treatment of severe IgA nephropathy in children. Pediatr Nephrol 3:248–253
Yamamoto-Shuda Y, Nakayama K, Saito T, Natori Y (1999) Therapeutic effect of glucocorticoid on experimental crescentic glomerulonephritis. J Lab Clin Med 134:410–418
Algoet C, Proesmans W (2003) Renal biopsy 2–9 years after Henoch–Schönlein purpura. Pediatr Nephrol 18:471–473
Tipping PG, Thomson NM, Holdsworth SR (1986) A comparison of fibrinolytic and defibrinating agents in established experimental glomerulonephritis. Br J Exp Pathol 67:481–491
Drew AF, Tucker HL, Liu H, Witte DP, Degen JL, Tipping PG (2001) Crescentic glomerulonephritis is diminished in fibrinogen-deficient mice. Am J Physiol Renal Physiol 281:F1157–F1163
Kitching AR, Kong YZ, Huang XR, Davenport P, Edgtton KL, Carmeliet P, Holdsworth SR, Tipping PG (2003) Plasminogen activator inhibitor 1 is a significant determinant of renal injury in experimental crescentic glomerulonephritis. J Am Soc Nephrol 14:1487–1495
Ono T, Muso E, Suyama K, Oyama A, Matsushima H, Yashiro M, Kuwahara T, Yoshida H, Kanatsu K, Sasayama S (1996) Intraglomerular deposition of cross-linked fibrin in IgA nephropathy and Henoch–Schönlein purpura nephritis. Nephron 74:522–528
Watanabe T, Takahashi S, Nakajo S, Hamasaki M (1996) Pathological improvement of IgA nephropathy and Henoch–Schönlein purpura nephritis with urokinase therapy. Acta Paediatr Jpn 38:622–628
Ronkainen J, Autio-Harmainen H, Nuutinen M (2003) Cyclosporin A for the treatment of severe Henoch–Schönlein glomerulonephritis. Pediatr Nephrol 18:1138–1142
Bohle A, Mackensen-Haen S, von Gise H (1990) The consequences of tubulo-interstitial changes for renal function in glomerulopathies: a morphometric and cytological analysis. Pathol Res Pract 186:135–144
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shin, J.I., Park, J.M., Kim, J.H. et al. Factors affecting histological regression of crescentic Henoch–Schönlein nephritis in children. Pediatr Nephrol 21, 54–59 (2006). https://doi.org/10.1007/s00467-005-2068-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-005-2068-5