Abstract
Unlike iron therapy, folate use is not a standard of care in hemodialysis (HD) patients. Despite iron repletion, poor response to erythropoietin (EPO) treatment is common. Theoretical evidence for folate deficiency (FD) includes chronic blood loss, inflammation, malnutrition, and nutrient loss during dialysis. Due to poor diagnostic standards, early studies failed to establish a role for FD in EPO resistance. Given that hematological response to therapeutic intervention is the gold standard for FD, its diagnosis was therefore based on composite scoring of RBC and/or folate indices.
Fifteen subjects (8–20 years) on chronic HD were enrolled in this study. No folate supplement was given in the first six months. Thereafter, 5-mg folic acid was administered orally after HD sessions over a six-month period. Folate indices before and after treatment were compared using percentage differences and paired t-tests. After folate use, the mean Hb increased by 11.4%, while MCV and RDW were reduced. Similarly, 4 of the 15 subjects each had a ≥20% rise in Hb and a ≥5% reduction in MCV, while 46.7% had a ≥2.5% reduction in RDW. Mean RBC folate increased by 24%, while FD scores reduced from 3.8±1.2 to 0.4±0.7, and the EPO requirement by 90%.
In contrast to previous studies, 26.7% of study subjects met the criteria for FD. Furthermore, the substantial (post-folate) reduction in the EPO requirement validates the need for therapeutic intervention, and therefore the presence of functional FD in the population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Anemia control in hemodialysis (HD) subjects reduces hospitalization rates and health care expenditures [1]. Quality of life, including physical performance, psychosocial and cognitive functions, are improved [2, 3]. Inadequate erythropoietin (EPO) production is the principal cause of anemia in chronic kidney disease (CKD) [4]. Consequently, the use of recombinant human EPO (rhEPO) has revolutionized its clinical management [3, 5].
Although adequate Fe repletion is an essential component of anemia therapy in CKD [6, 7], routine folate supplementation is not a standard of care in HD. However, there is a wide variability in the effective therapeutic dose of EPO, despite adequate Fe status [8]. The etiology of EPO resistance may include folate deficiency (FD), chronic blood loss, oxidative stress, inadequate dialysis and malnutrition [8, 9, 10, 11, 12, 13, 14]. Others are TNF-α cytokines, hyperparathyroidism, ACE genotype polymorphism and uremic retention of hematopoietic inhibitor [13, 14, 15, 16, 17]. Whereas most of these factors are poorly modifiable, FD is easy to control at a relatively low cost.
Folic acid is required for DNA synthesis during erythropoiesis [9]. Dialysis patients are at risk of FD from poor nutrition, shortened red blood cell (RBC) lifespan, and hydrosoluble nutrient loss [9, 10, 11]. Folate loss during HD may be exacerbated by high-flux membranes, with a drop in serum folate (SF) within two months of treatment [10, 11].
The paucity of data on the role of FD in EPO resistance may be due to the poor specificity of folate diagnostic indices [18, 19, 20]. Serum folate varies widely with dietary intake and is also underestimated in CKD due to retention of folate binding proteins [19, 20]. In addition, although RBC folate has a better correlation with tissue folates, its normal reference values vary widely [20]. Furthermore, the diagnostic accuracy of serum and RBC folates are genetically influenced by (metabolizing) enzyme polymorphisms, the laboratory techniques used, and the geographical location [21, 22, 23, 24].
In non-dialysis subjects, an early diagnosis of cellular folate depletion can be made by estimating total plasma homocysteine [12]. Unfortunately, its accuracy in ESRD is confounded by its plasma retention [25]. Despite its subjectivity, therapeutic response to folate therapy is still favored as the most accurate means of FD diagnosis [12]. Thus, unlike the use of laboratory diagnostic indices, FD was defined by composite scoring of the quantitative changes in RBC indices after folate use.
Objectives
The objectives of the study were:
-
i.
To determine the prevalence of folate deficiency in children and adolescents on chronic HD.
-
ii.
To examine the impact of folate administration on anemia control in the study population.
-
iii.
To examine the role of folate deficiency on impaired EPO response in the study subjects.
Subjects and methods
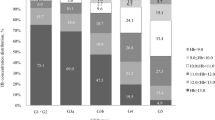
A total of 15 subjects who had received HD for a minimum of 3 months and rhEPO for 2 months between the period of July 2002 and July 2003 were enrolled for the study. Their mean age was 16±3.5 yrs with a range of 8–20. The male to female ratio was 3:2. Five of the 15 subjects had focal segmental glomerulosclerosis (33.3%), 4 had renal dysplasia (26.6%), 2 had non-specific chronic glomerulonephritis (13%), 1 patient each had membranoproliferative glomerulonephritis (6.6%), HIV-associated nephropathy (6.6%), tubulo-interstitial nephropathy (6.6%) and lupus nephritis (6.6%). There were 10 Hispanics and 5 African Americans, largely reflecting the demographic characteristics of the hospital patient population. All patients had normal hemoglobin (Hb) genotypes except for Hb AS in an African American. Twelve subjects (80%) routinely used cuffed double-lumen HD catheters for vascular access while three had native arteriovenous fistulas.
Prior to the initiation of the study, no patient was on routine folate supplementation. Baseline complete blood count (CBC), serum and RBC folates, serum vitamin B12, and total plasma homocysteine (tHcy) were obtained. However, there was an overlap in the diagnostic accuracy of the RBC parameters. Thus, mean corpuscular volume (MCV) value may be indicative of FD in one patient, while random distribution width (RDW) may suggest otherwise. Therefore, in order to enhance their predictive values, the FD was defined by aggregate scores of the RBC indices. Similarly, FD (score) was quantified by a composite score of MCV, RBC morphology, serum and RBC folates. The FD score was validated previously in a pilot survey of the same population. In addition, EPO resistance index (ERI) was calculated by dividing the values of the hematocrit (%) by the corresponding weekly EPO dose (unit-dose per kg body weight). Observer-confirmed oral doses of 5-mg folates were given three times per week at the end of the HD session. Anemia was managed according to NFK/DOQI guidelines [6]. The dose of rhEPO was increased by 25% every 2–4 weeks to achieve a target Hb of 12.0 g/dl. Ten doses of 100–125 mg intravenous (IV) Fe were given with consecutive HD if Fe saturation was <20% and/or serum ferritin was <100 ng/mL. If Fe saturation was >20% and/or ferritin was >100 ng/mL, IV Fe (25–125 mg) was administered weekly. If Fe saturation was >50% and/or ferritin was >800 ng/mL, Fe therapy was withheld for up to 3 months.
Laboratory techniques
Plasma homocysteine was measured using the fluorescence polarization immunoassay (FPIA) method (Abbott Laboratories, Abbott Park, IL, USA) while serum vitamin B12, serum and RBC folates were analyzed by Stratus fluorometric enzyme-linked assay (DADE-Behring, Deerfield, IL, USA).
Statistics
All data sets were analyzed for a Gaussian distribution using the D’Agostino-Pearson test for kurtosis, skewness and omnibus normality. Normally distributed data were reported as means ± standard deviations (SD). Otherwise the data were expressed as medians and ranges. Folate indices obtained during the first six months were compared to the corresponding (post-folate) values using paired t-tests (for parametric) and Wilcoxon-Signed Rank sum tests (non-parametric). In addition, due to the small number of study subjects, all normally distributed data were further analyzed with non-parametric statistics. However, there was no disagreement among the results obtained from the two analyses, so only parametric equivalents were reported.
The hematological impact of folate therapy was estimated by comparing (by paired t-test) the sequential changes (or Δ) of the monthly Hb, MCV, EPO doses and ERI before and after folate use. Pre- and post-treatment changes in folate indices were further analyzed after sub-classification of subjects into FD and non-FD groups. Additionally, temporal effects of folate use were assessed by comparing the changes in RBC and EPO (resistance) indices for the first and the last three months of therapy.
Indices for folate status, malnutrition and inflammation were scored as composites in order to minimize the effects of confounders (see Appendix A). In addition, the mean body mass index (BMI) was analyzed at the onset and after 6 months and 12 months of the study, using a two-by-three factorial ANOVA. The FD was defined by composite scoring of hematological responses to folate use (see the FD diagnosis parameters described below). A p value ≤0.05 was considered significant for all statistics.
Composite scoring scheme
Folate deficiency scores:
-
a.
RBC folate≤2×SD (score=2)
-
b.
MCV≥95th percentile for age and sex (=2) [26]
-
c.
Megaloblastic RBC on blood film or RDW≥16 (=1)
-
d.
Hb≤11 g/dL (with Fe saturation ≥20% and serum ferritin ≥100 mg/dL) (=1)
The scoring system was presumed valid only in the presence of a normal serum vitamin B12.
Malnutrition inflammatory score (MIS) [27]. Comprehensive MIS (CMIS) is a questionnaire designed to quantify nutritional deficiency and inflammatory burdens, and it has been validated for adult dialysis patients [27]. Its components are weight change, dietary intake, functional impairment, comorbidity, loss of fat mass, serum albumin and transferrin. To enhance its applicability to a pediatric population, objective MIS (OMIS) was adapted from CMIS. Its scoring is devoid of the subjective items in CMIS (Appendix A).
Folate deficiency parameters
Folate deficiency was defined based on the following hematological responses to folate therapy:
-
a.
Twenty percent increase in the mean monthly Hb obtained during folate use over the baseline (mean Hb during the previous six months), and
-
b.
Five percent decrease in the mean monthly MCV over the baseline, and/or
-
c.
Two and one-half percent reduction in the mean monthly RDW below the baseline.
Thus a patient is deemed to be folate-deficient if he meets at least 2 of the 3 criteria including item (a).
Results
Red blood cell indices
As illustrated by Table 1, the mean Hb for all subjects prior to folate use (PreTX) was 10.5 g/dL. Its value increased by 8% after treatment (p=0.03). Similarly, with folate use, four of the subjects had a ≥20% rise in Hb values, while two patients exhibited an increase of 15–19%. Furthermore, there was 1.8% reduction in the mean MCV (p=0.05) and a 3.3% reduction in RDW (p=0.001). Twenty-six percent of the 15 subjects had ≥5% reduction in mean MCV, while 46.7% had ≥2.5% reduction in RDW.
Folate and vitamin B12 indices
As demonstrated by Table 2, after six months of treatment the mean serum folate had increased by 38% (p=0.001), while RBC folate appreciated by 24% (p=0.02). There was an 850% reduction in FD score (p=0.001). In contrast, serum vitamin B12 increased by only 5.6% (p=0.2), while plasma homocysteine depreciated by 12% (p=0.2).
Prevalence of folate deficiency
Twenty-six percent (patients 2, 6, 7 and 11) of the subjects (Table 1) met the criteria for FD, which included ≥20% increase in mean Hb, ≥5% decrease in MCV and/or ≥2.5% reduction in RDW in response to folate use.
Hematological impact of folate use
Pre- and post-folate treatments
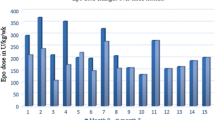
As illustrated by Table 3, the sum total of the mean Δ of monthly Hb, MCV, EPO doses and the EPO resistance index before and after folate use were compared. There was a 2.5 g/dL drop in the mean Hb before folate treatment, followed by a positive gain after therapy. Similarly, an increase in mean ΔMCV of 4 fL was observed prior to folate use, followed by a cumulative loss of 7 fL after treatment. Whereas EPO doses increased in the absence of folate, after treatment there was a net reduction in requirement. Furthermore, there was a significant change in the mean ΔEPO resistance index before and after folate use. Although changes in the mean RBC indices after three months of folate use were not significant (Fig. 1), substantial differences were noted by the second half of treatment: Hb (p=0.01), MCV (p=0.02) and RDW (p=0.001). While there was a 57% increase in ERI in the first 3 months (p=0.04), a 180% increase was seen by the second half of the study (p=0.0001).
Folate- (FD) and non-folate-deficient (NFD) subjects
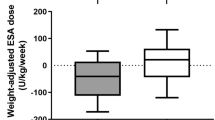
We further analyzed the study population (Table 4) by comparing those with FD to those without (NFD). Prior to folate use, the mean serum folate was nearly equal in both groups (7.8 vs. 8.1), but with treatment a substantial increase was noted in both FD (p=0.04) and NFD (p=0.01). Whereas the baseline RBC folate was lower in FD (379 vs. 633), after treatment there was an increase of 240 units (p=0.05) in FD and 150 units (p=0.1) in NFD. While pre-folate homocysteine was higher in FD (40.2 vs. 17.3; p=0.0001), there was a 52% reduction in FD and a 43% reduction in NFD after treatment (19 vs. 10; p=0.7). Although the mean serum vitamin B12 was lower prior to folate use in FD (367 vs. 694; p=0.01), with treatment there was no significant increase (p=0.1 vs. p=0.4) in both groups (Table 4). In the absence of folate, the mean ERI for NFD was twice (1.6 vs. 0.8) that of FD (p>0.05); there was a significant increase in ERI after folate use in both groups.
Analysis of confounding variables
Iron indices
Following folate use, the (median) monthly serum Fe, serum ferritin, Fe saturation and serum transferrin were 66 μg/dL (95% CI=61–84), 499.5 mg/dL, 41.5% (Table 5) and 162 mg/dL (95% CI=74–1508) respectively. Similarly, the median Fe saturation was ≥20% in all subjects and median serum ferritin was ≥100 mg/dL in 14 patients. Furthermore, there was no significant difference in the mean Fe saturation (41.4±4 vs. 37±8; p=0.36) and the mean doses of IV Fe (sodium ferric gluconate) before and during folate use (250±58 mg vs. 244±84 mg; p=0.45).
Serum calcium, phosphates and intact PTH
As shown by Table 5, the median Ca and P products for all subjects was 49 mg2/dL2. The median intact PTH was 688 pg/mL (38.5–2000). Patient 1 recorded the highest value of PTH due to poor adherence to medical care. There was no significant difference in mean serum Ca and P products (41.0±10 vs. 51.2±10 mg2/dL2) and intact PTH (652±647 vs. 907.3±650) in FD and NFD.
Dialysis adequacy, nutritional and inflammatory indices
The (median) monthly urea reduction ratio (URR) for all subjects was 70.4%, while the median for Kt/V was 1.5. During folate use, 93.3% of the population had a mean URR≥65% and Kt/V≥1.2. The median c-reactive protein was 0.8 mg/dL (0.1–4.6 mg/dL), while median serum albumin was 4.1 g/dL (1.5–4.6 g/dL). Despite its limitations in dialysis subjects, body mass index (BMI) was calculated from the estimated dry weight for each patient. Statistical analysis (ANOVA) of the mean BMI at zero, six and twelve months of the study period showed no significant differences (p=0.11). Malnutrition and inflammation burden were graded by composite scores (OMIS and CMIS). The correlation coefficient between the CMIS and OMIS was 0.9 (99% CI=0.7–1) and p was <0.0001. There was no significant difference in the mean OMIS (10.2±3 vs. 8.1±2), and CMIS (9.0±3 vs. 7.3±2) for FD and NFD (p=0.24).
Cost analysis
Over the six months of folate use, lower doses of epoetin alfa or Aranesp (19,311.15 units per week) were required (Table 3). At a cost of $281.55 for 10,000 units, the total amount saved per subject was $870, and for all patients was $13,050.
Discussion
Twenty-six percent of the study subjects met the criteria for FD, contrary to previous reports of folate sufficiency in the dialysis population. The prevalence of FD is often under-reported when diagnosis is based on laboratory references of serum and RBC folates. Thus, in a cross-sectional study of adult HD subjects, only 1.8% with low RBC folate were considered folate-deficient, whereas 63% had subnormal serum folate [28]. However, in children with CKD, the mean serum folate was lower in the subjects than in the controls [29]. Similarly, in Israeli adults on HD, although 80% had a low serum folate, FD was suggested by the lower EPO doses required to maintain higher Hb levels [30].
In general, the outcomes of FD (prevalence) studies are determined by the study designs. The current data were derived from a twelve-month longitudinal (cohort) study that compared the first half (without folate use) and the last six months of treatment. The therapeutic response of the patient to folate use was then analyzed by examining changes in EPO requirement, RBC and folate indices. Because each subject serves as his/her own control, confounding effects were minimized. Furthermore, the potential bias from differences between the study group and the laboratory reference population was avoided.
Prior to folate use, the median MCV and RDW for most subjects were greater than the 75th percentile for age (Table 1) and the mean Hb was less than the minimum recommended by K/DOQI (11 g/dL) [1, 5, 6]. Following treatment, there was reduction in MCV and RDW and an increase in mean Hb to 11.4 g/dL. Similarly, despite the normal (compared with laboratory reference) baseline folate in all subjects, FD was implied by a significant increase in post-folate values (Table 3). On the other hand, there was a very little change in pre- and post-folate serum vitamin B12.
Furthermore, although MCV and RBC morphology are more accurate determinants of FD than the folate indices [31, 32], MCV may be confounded by increased peripheral erythroblasts in response to EPO therapy [9]. In addition, RDW, a measure of erythrocyte volumetric variation, is not specific for FD [9]. On the other hand, while a low serum folate may suggest FD, a normal value does not necessarily imply adequate folate stores [19, 20]. Given these limitations, RBC and folate indices were scored (as a composite) to provide a quantitative estimate of folate status (FD Score). The danger of over-reliance on the diagnostic value of these laboratory indices was exemplified by the poor therapeutic response of patient 5 to folic acid (non-FD) despite the high initial FD score (Table 2). In contrast, patient 2 met the criteria for folate deficiency regardless of the lower FD score.
The impact of folate intervention on RBC indices and EPO requirements was estimated by the differences in their Δ values before and after folate use. As shown in Table 3, before treatment, there was a net drop in the Hb value and an increase in the MCV and EPO requirements. After folate use, the trend was reversed. In addition, the (post-folate) increase in ERI is a clear indication of a lower EPO requirement. The cost analysis showed a net saving of $870 per subject during the folate therapy. Similar cost savings have been previously demonstrated with the combined use of parenteral iron and EPO [1, 7, 33]. Similarly, since the peak hematological response to folate takes about 6–8 weeks, its impact is expected to increase progressively with treatment. Consequently, the magnitude of change in Hb, MCV, RDW and EPO requirement was greater in the last three months of folate use than during the initial period (Fig. 1).
Furthermore, after classification of the subjects into FD and non-FD, the therapeutic responses to folate were found to be more substantial in the FD group (Table 4); a reasonable indicator of the internal validity of the FD diagnostic parameters. In addition, pre-folate homocysteine, an established cardiovascular risk factor in the non-renal population, was higher in FD than in the non-FD subjects, reflecting the common finding of hyperhomocysteinemia in FD states (Table 4) [25, 34, 35, 36].
The potential impact of confounding variables was either controlled during the study design and/or accounted for in the analysis. To avoid Fe deficiency anemia, all patients received IV Fe, as recommended by NKF/DOQI [7, 37]. Doses of IV iron given before and after folate use were not significantly different. Iron indices suggest adequate Fe repletion in all subjects. Although hyperparathyroidism was universal in the study group, the Ca and P product was below 70 in 14 of the patients. However, the remarkably poor hematological response to folate in patient 1 (with highest PTH) may result from severe hyperparathyroidism. The paradoxical drop in EPO dose in the same patient despite the reduction in Hb may be due to its erratic administration resulting from a poor adherence to dialysis schedule by the subject (Table 1). This observation was further corroborated by the wide variance in the mean EPO requirement (37.5±20) during the first six months of the study. Nearly all subjects (93%) met the target URR of 65% and Kt/V of 1.2. Furthermore, there was no significant difference between the malnutrition-inflammation scores in both FD and NFD groups. This is also true for Fe indices, BMI, Ca, P, and intact PTH (Table 5).
In summary, the study supports the routine use of folate for effective management of anemia in the HD population. In the absence of a diagnostic standard, aggregate scoring of surrogate indices was used. In contrast to the previous reports of folate sufficiency, 26.7% of the cohort met the criteria for FD. However, because most patients had some therapeutic response to folate use, the FD sub-group may well represent those with the most severe deficiencies. Thus the true prevalence of FD may be higher than reported. Furthermore, the need for folate use was validated by the substantial reduction in the EPO requirement after folate therapy.
The small sample size limited the application of multiple regression in the analysis of confounding variables [38]. In addition, because the diagnostic instruments were designed for the study population, there is need for caution when generalizing some of the findings. Nevertheless, the favorable outcome of our study calls for utilization of a similar methodology in future studies.
Appendix A
Objective Malnutrition Inflammation Score (OMIS). The OMIS is a questionnaire designed to quantify nutritional deficiency and inflammatory burdens. Component indices and parameters are scored as shown below.
a) Primary renal pathology:
- Score of 0::
-
Non-inflammatory (e.g. dysplasia)
- 2::
-
Somewhat inflammatory (e.g. FSGS, previous renal transplant)
- 3::
-
Inflammatory (e.g. lupus nephritis, HIV nephritis)
b) Serum albumin:
- Score of 0::
-
≥4 g/dl
- 1::
-
3.5–3.9 g/dl
- 2::
-
3.0–3.4 g/dl
- 3::
-
<3.0 g/dl
c) Serum total Fe binding capacity:
- Score of 0::
-
≥250 mg/dl
- 1::
-
200–249 mg/dl
- 2::
-
150–199 mg/dl
- 3::
-
<150 mg/dl
d) Body Mass Index:
- Score of 0::
-
≥20 kg/m2
- 1::
-
15–19.9 kg/m2
- 2::
-
16–17.9 kg/m2
- 3::
-
<16 kg/m2
e) Dialysis duration:
- Score of 0::
-
0–1 yrs
- 1::
-
1–3
- 2::
-
3–5
- 3::
-
>5
f) Grade of infection:
- Score of 0::
-
None
- 0.5×(number of events)::
-
low-grade (e.g. line induced bacteremia, upper respiratory tract infection)
- 1.0×(number of events)::
-
moderate (e.g. symptomatic line sepsis, pneumonia)
- 1.5×(number of events)::
-
severe (infection warranting hospitalization)
g) Dialysis adequacy:
- Score of 0::
-
URR≥80%
- 1::
-
61–79%
- 2::
-
41–60%
- 3::
-
<40%
h) Serum C-reactive protein:
- Score of 0::
-
0–0.9 mg/dl
- 1::
-
1–1.9 mg/dl
- 2::
-
2–4.9 mg/dl
- 3::
-
>5 mg/dl
i) Serum ferritin:
- Score of 0::
-
0–500 mg/dl
- 1::
-
501–750 mg/dl
- 2::
-
751–1000 mg/dl
- 3::
-
>1001 mg/dl
References
Collins AJ, Li S, St Peter WL, Ebben J, Roberts T, Ma JZMW (2001) Death, hospitalization, and economic associations among incident hemodialysis patients with hematocrit levels of 36–39%. J Am Soc Nephrol 12:2465–2473
Ross SD, Fahrbach K, Frame D, Scheye R, Connelly JE, Glaspy J (2003) The effect of anemia treatment on selected health-related quality of life domains: a systematic review. Clin Ther 25:1786–1805
Revicki D, Brown R, Feeny D, Henry D, Teehan B, Rudnick M, Benz R (1991) Health related quality of life with human erythropoietin treatment for predialysis chronic renal disease patients. Am J Kidney Dis 25:548–554
Eschbach JW, Varma A, Stivelman JC (2002) Is it time to for a paradigm shift? Is erythropoietin deficiency still the main course of renal anaemia? Nephrol Dial Transplant 17(Suppl 5):2–7
The US Recombinant Human Erythropoietin Predialysis Study Group (1991) Double-blind, placebo-controlled study of therapeutic use of recombinant human erythropoietin for anemia associated with chronic renal failure in predialysis patients. Am J Kidney Dis 18:50–59
National Kidney Foundation (2000) K/DOQI clinical practice guidelines for anemia of chronic kidney disease. Am J Kidney Dis 37:S182–S238
Besarab A, Amin N, Ahsan M, Vogel SE, Zazuwa G, Frinak S, Zazra JJ, Anandan JV, Gupta A (2000) Optimization of epoietin therapy with intravenous iron therapy in hemodialysis patients. J Am Soc Nephrol 11:530–538
Eschbach JW, Abdulhadi MH, Browne JK, Delano BG, Downing MR, Egrie JC, Evans RW, Friedman EA, Graber SE, Haley NR, et al (1989) Recombinant human erythropoietin in anemic patients with end stage renal disease. Results of phase II multicenter clinical trial. Ann Intern Med 111:992–1000
Allen RH (2000) Megaloblastic anemias. In: Goldman L (ed) Cecil textbook of medicine, 21st edn. WB Saunders Co, Philadelphia, PA
Leblanc M, Pichette V, Geadahy D, Ouimet D (2000) Folic acid and pyridoxal-5′-phosphate losses during high-efficiency hemodialysis in patients without hydrosoluble vitamin supplementation. J Ren Nutr 10:196–201
Lasseur C, Parrot F, Delmas Y, Level C, Ged C, Redonnet-Vernhet I, Montaudon D, Combe C, Chauveau P (2001) Impact of high-flux/high-efficiency dialysis on folate and homocysteine metabolism. J Nephrol 14:32–35
Galluci MT, Lubrano R, Meloni C, Morosetti M, Manca di Villahermosa S, Scoppi P et al (1999) Red blood cell membrane lipid peroxidation and resistance to erythropoietin therapy in hemodialysis patients. Clin Nephrol 52:239–245
Cooper AC, Mikhail A, Lethbridge MW, Kemeny DM, MacDougall IC (2003) Increased expression of erythropoiesis inhibiting cytokines (IFN-gamma, TNF-alpha, IL-10, and IL-13) by T cells in patients exhibiting a poor response to erythropoietin therapy. J Am Soc Nephrol 14:1776–1784
Lin CL, Huang CC, Yu CC, Wu CH, Chang CT, Hsu HH, Hsu PY, Yang CW (2002) Improved iron utilization and reduced erythropoietin resistance by on-line hemodiafiltration. Blood Purif 20:349–356
Varagunam M, McCloskey DJ, Sinnot PJ, Raftery MJ, Yaqoob MM (2003) Angiotensin-converting enzyme gene polymorphism and erythropoietin requirement. Perit Dial Int 23:111–115
Seeherunvong W, Rubio L, Abitbol CL, Montane B, Strauss J, Diaz R et al (2001) Identification of poor responders to erythropoietin among children undergoing hemodialysis. J Pediatr 138:710–714
Le Meur Y, Lorgeot V, Comte L, Szelag JC, Aldigier JC, Leroux-Robert C, Praloran V (2001) Plasma levels and metabolism of AcSDKP in patients with chronic renal failure: relationship with erythropoietin requirements. Am J Kidney Dis 38:510–517
Hershko C, Grossowicz N, Rachmilewitz M, Kestern S, Izak G (1975) Serum and erythrocyte folates in combined iron and folate deficiency. Am J Clin Nutrition 28:1217–1222
Paine CJ, Hargrove MD Jr, Eichner ER (1976) Folic acid binding protein and folate balance in uremia. Arch Int Med 136:756–760
Phekoo K, Williams Y, Schey SA, Andrews VE, Dudley JM, Hoffbrand AV (1979) Folate assays: serum or red cell? J R Coll Physicians Lond 31:291–295
Shelnutt KP, Kauwell GP, Chapman CM, Gregory JF 3rd, Maneval DR, Browdy AA, Theriaque DW, Bailey LB (2003) Folate status response to controlled folate intake is affected by the methylenetetrahydrofolate reductase 677C-->T polymorphism in young women. J Nutr 133:4107–4111
Fodinger M, Dierkes J, Skoupy S, Rohrer C, Hagen W, Puttinger H, Hauser AC, Vychytil A, Sunder-Plassmann G (2003) Effect of glutamate carboxylase II and reduced folate carrier polymorphisms on folate and total homocysteine concentrations in dialysis patients. J Am Soc Nephrol 14:1314–1319
Owen WE, Roberts WL (2003) Comparison of five automated serum and whole blood folate assays. Am J Clin Pathol 120:121–126
Philpott N, Kelleher BP, Smith OP, O’Broin SD (2001) High serum folates and the simplification of red cell folate analysis. Clin Lab Haematol 23:15–20
Lilien M, Duran M, Van Hoeck K, Poll-The BT, Schroder C (1999) Hyperhomocyst(e)inaemia in children with chronic renal failure. Nephrol Dial Transplant 14:366–368
Hudspeth M, Symons H (2002) Hematology. In: Gunn VL, Nechyba C (eds) The Harriet Lane handbook, 16th edn. Mosby Inc, Philadelphia, PA
Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH (2001) A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis 38:1251–1263
Bamonti-Catena F, Buccianti G, Porcella A, Como G, Finnazzi S, Malolo AT (1999) Folate measurements in patients on regular hemodialysis treatment. Am J Kidney Dis 33:492–497
Merouani A, Lambert M, Delvin EE, Genest J, Robitaille P, Rozen R (2001) Plasma homocysteine concentration in children with chronic renal failure. Pediatr Nephrol 16:805–811
Korzets A, Ori Y, Chagnac A, Weinstein T, Herman M, Zevin D, Malachi T, Gafter U (2000) Erythropoietin, folic acid deficiency and hyperhomocysteinemia: is there a possible relationship in chronically hemodialyzed patients? Clin Nephrol 53:48–54
Haltmayer M, Mueller T, Poelz W (2002) Erythrocyte mean cellular volume and its relation to serum homocysteine, vitamin B12 and folate. Acta Med Austriaca 29:57–60
Savage DG, Ogundipe A, Allen RH, Stabler SP, Lindenbaum J (2000) Etiology and diagnostic evaluation of macrocytosis. Am J Med Sci 319:343–352
Sheingold S, Churchill D, Muirhead N, Laupacis A, Labelle R, Goeree R (19929 The impact of recombinant human erythropoietin on medical care costs for hemodialysis patients in Canada. Soc Sci Med 34:983–991
Martin I, Obrador A, Gibert MJ, Hernanz A, Fuster A, Pintos C, Garcia A, Tur J (2003) Folate status and a new repletion cut-off value in a group of healthy Marjocan women. Clin Nutr 22:53–58
Farid FRA, Faheem MS, Heshmat NM, Shaheen KY, Saad SS (2004) Study of homocysteine status in children with chronic renal failure. Am J Nephrol 24:289–295
Kang HG, Lee BS, Hahn H, Lee JH, Ha IS, Cheong HI, Choi Y (2002) Reduction of plasma homocysteine by folic acid in children with chronic renal failure. Pediatr Nephrol 17:511–514
Van Wyck DB (2000) Management of early renal anaemia: diagnostic work up, iron therapy, epoietin therapy (review). Nephrol Dial Transplant 15(Suppl 3):36–39
Grimes DA, Schulz KF (2002) Bias and causal associations in observational research (review). Lancet 359:248–252
Acknowledgements
This study was presented in abstract form at the Pediatric Academic Society Annual Meeting, Washington DC, May 2005. Oluwatoyin Fatai Bamgbola was supported by NIDDK Training Grant No. 9-526-3740. We appreciate the assistance of Dr. Martin Turman, Dr. James Wenzl, Dr. Kathleen O’Neil of Department of Pediatrics, Oklahoma University Health Science Center, OK, USA, for their assistance in proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bamgbola, O.F., Kaskel, F. Role of folate deficiency on erythropoietin resistance in pediatric and adolescent patients on chronic dialysis. Pediatr Nephrol 20, 1622–1629 (2005). https://doi.org/10.1007/s00467-005-2021-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-005-2021-7