Abstract
Children with steroid-dependent minimal change nephrotic syndrome are prone to serious steroid side effects. Alternative therapies, such as oral cyclophosphamide, may also have serious side effects. We conducted this novel prospective study to compare the long-term efficacies of levamisole and I.V. pulse cyclophosphamide as therapies with potentially fewer side effects. This study included 40 children with idiopathic steroid-dependent minimal change nephrotic syndrome (age 3–15 years; 31 boys and 9 girls). The patients were randomized into two equal groups. One group received levamisole 2.5 mg/kg on alternate days (levamisole group) while the other group received I.V. cyclophosphamide 500 mg/m2/month for six months (cyclophosphamide group). Prednisolone was gradually withdrawn. After stopping treatment, the number of patients that maintained remission was five (25%) in each group at six months, four (20%) versus two (10%) at one year and three (15%) versus one (5%) at two years in the levamisole and cyclophosphamide groups respectively, and one (5%) in each group at three and four years. The overall side effects were mild and both drugs were well tolerated. In view of the results, we recommend trial of levamisole before adopting other therapies with more serious side effects in such patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Minimal change nephrotic syndrome (MCNS) accounts for 80% of idiopathic nephrotic syndrome in children. Although 93% of patients will be steroid-responsive, only 38% of the primary responders will be non-relapsers, while the remaining will display a relapsing course and commonly acquire steroid dependency. Steroid-dependent patients are prone to serious steroid side effects, such as growth retardation, peptic ulceration, cataracts, osteoporosis, aseptic necrosis of the femoral head, acne, moon facies and psychiatric disturbances. Thus, the physician may attempt adjunctive therapy in such patients [1, 2]. Oral cyclophosphamide is usually recommended in this situation. However, long-term side effects like gonadal toxicity become an important issue [3]. I.V. cyclophosphamide pulse administration may lead to a reduction in total dose compared to daily oral treatment. Therapy with I.V. cyclophosphamide pulses is therefore an emerging therapeutic option in an attempt to maintain remission with less frequent side effects [4]. On the other hand, the long-term immunosuppression in such patients and the cumulative risk of further alkylating therapy are worrying in a condition that ultimately has an excellent prognosis. Thus, alternative therapy with the immunostimulant agent levamisole would be another attractive option, especially in MCNS, which is a condition characterized by altered cellular immunity [5].
Only one study has retrospectively compared levamisole with oral cyclophosphamide in frequently relapsing steroid-dependent nephrotic syndrome, and it concluded that levamisole may be considered an alternative for oral cyclophosphamide as a second-line agent after corticosteroids in such patients [6]. However, to our knowledge, no studies have compared the efficacy and safety of levamisole with that of I.V. cyclophosphamide pulse therapy as two therapeutic options with potentially fewer side effects in steroid-dependent MCNS children. Therefore, we conducted this prospective random study to accomplish this goal.
Methods
Subjects
This prospective study included 40 children with idiopathic nephrotic syndrome selected from patients attending the outpatient clinic or admitted to the nephrology department of our center in the period between May and December 1998. Their ages ranged from 3 to 15 years and they comprised 31 boys and 9 girls. The initial corticosteroid protocol for all of them at the start of the disease was the ISKDC protocol (short attack treatment). All of them had a steroid-dependent pattern of response, defined as occurrence of complete remission on steroids but relapse while withdrawing or within two weeks after discontinuing steroid treatment [7]. Also, all of them had biopsy-proven minimal change lesions. Renal biopsies were performed before the start of the study. During this period, our protocol was to biopsy children with steroid-dependent nephrotic syndrome for whom adjunctive therapy will be added. Nevertheless, kidney biopsies were performed for all patients only after steroid dependency was established. All patients received more than one steroid course. None of our children had received any type of adjunctive therapy for their nephrotic status before the study. All patients had normal creatinine clearance corrected to surface area [8], normal liver function tests and normal complete blood count at the start of the study. Parental consent was obtained before the study, and the scientific and ethics committee of the hospital approved the study.
Study design
A prospective and randomized study was adopted. The patients were randomized into two equal groups each containing 20 patients. One group was allocated to receive oral levamisole (levamisole group) while the other group was allocated to receive I.V. cyclophosphamide (cyclophosphamide group).
All patients were in relapse at the start of the study. This relapse qualified them for recruitment to the study and was treated by increasing the prednisolone dose to 2 mg/kg/day until remission (protein-free urine on three consecutive days) followed by decreasing this to 1 mg/kg on alternate days for 14 days. At that point, either levamisole or IV cyclophosphamide pulse therapy was started. Levamisole was given for patients in the levamisole group at a dose of 2.5 mg/kg on alternate days (the same days as the steroid dose). For patients in the cyclophosphamide group, cyclophosphamide was given in a dose of 500 mg/m2/month. The calculated cyclophosphamide dose was diluted in 100 cc of 5% dextrose and given by I.V. infusion over a period of one hour. During I.V. cyclophosphamide infusion, patient parameters (pulse, blood pressure and temperature) as well as any symptoms (such as nausea and vomiting) were monitored. After the infusion was finished, 250–500 cc of 5% dextrose or 0.9% sodium chloride was given rapidly I.V. and the patient was asked to consume an ample amount of fluids and void frequently for the rest of the day [9]. After the addition of either levamisole or cyclophosphamide, steroids were continued at 1 mg/kg on alternate days for a further 14 days and then reduced by 0.25 mg/kg every 14 days until complete withdrawal. Thus, steroids had been discontinued at two months following the start of levamisole or cyclophosphamide. The steroid protocol used in the study was similar to that used for treatment of previous relapses prior to inclusion in the study.
Levamisole and cyclophosphamide were continued for 6 months, as long as remission was maintained, but was stopped if relapse occurred at any time during the treatment period. Relapse was defined as protein positive urine of +++ for 3 consecutive days [5]. This result was confirmed by a 24-hour urinary protein >50 mg/kg [10].
Patients were followed up monthly. At each visit, clinical assessment, urinalysis, 24-hour urinary protein, complete blood count, serum creatinine, liver function tests and serum cholesterol were performed for all patients. In addition, parental monitoring was performed during inter-visit periods based on reappearance of edema, decreased urine output, or observation of any complications. In such cases, unscheduled hospital visits were performed. Parental monitoring was done for some children by performing urine heat tests for protein.
The response to either levamisole or cyclophosphamide was evaluated in terms of remission, change in the steroid response status of the patient, duration of remission, side effects, and compliance with therapy.
Statistical analysis
Continuous data of normal distribution were expressed as means±SD. An unpaired T-test was then performed to compare values in both groups. Continuous non-homogeneous values that showed deviation from the normal distribution were expressed as medians. A non-parametric statistical method (Mann-Whitney test) was then performed to compare values in both groups. For discrete values (frequencies), the cross-tabulation chi-square test was used to compare values in both groups.
Results
Table 1 shows that both groups were comparable regarding baseline characteristics at the start of the study. Hypertension was present in 40% and 35% of children in the levamisole and cyclophosphamide groups respectively and was defined as diastolic blood pressure above the 95th percentile for age, sex and height [11]. Three patients in the levamisole group were obese, as defined by body weight above the 95th percentile for age and sex [12]. Cushingoid facies was noticed in 25% of children in the levamisole group compared to 30% in the cyclophosphamide group at the start of the study. In both groups, 65% of the children were at or above the 50th percentile for height at the start of the study.
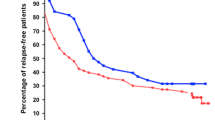
The number of patients in both groups who maintained remission was comparable throughout the study (Fig. 1). During the 6-month period of adjunctive treatment, 11 of 20 children (55%) in the levamisole group versus 10 out of 20 children (50%) in the cyclophosphamide group were able successfully to stop steroids for more than two weeks (they were no longer steroid-dependent). Throughout the rest of the study period, the duration of steroid-free remission was comparable in both groups (median/range of 6.83/48.73 months in the levamisole group versus 5.2/40.57 months in the cyclophosphamide group).
At the end of the 6-month period of adjunctive therapy (4 months after steroid discontinuation), 10 patients (50%) and 9 patients (45%) were in remission on levamisole and cyclophosphamide alone respectively.
During long-term follow-up after withdrawal of levamisole or cyclophosphamide therapy, the number of patients that maintained treatment-free remission was 5 (25%) in each group at 6 months, 4 (20%) versus 2 (10%) at 1 year, 3 (15%) versus 1 (5%) at 2 years and 1 (5%) in each group at 3 and 4 years.
Table 2 shows that the side effects that occurred during levamisole or cyclophosphamide therapy were comparable. The most common side effect was infection, which occurred in 65% and 60% of patients in the levamisole and cyclophosphamide groups respectively. However, all infections were mild and occurred during the period of combined corticosteroids and adjunctive therapy. Respiratory tract infections were all in the form of mild acute bronchitis. Asymptomatic pyuria occurred in two and four patients of the levamisole and cyclophosphamide groups respectively, but urine culture was positive in only one patient in each group. In the levamisole group, this patient had a history of recurrent urinary tract infection, and radioisotope study revealed the presence of coincidental grade one right vesicoureteric reflux. One patient in the levamisole group had personality changes, in the form of aggression and nervousness, noticed by parents and schoolteachers, after the commencement of steroid therapy. Mild alopecia was noticed in 15% of the cyclophosphamide group patients and it rapidly reversed after discontinuing cyclophosphamide. Hepatotoxicity in the form of mild and transient elevation of ALT and AST (65/42 IU/L; N=8–40 IU/L) occurred in only one patient in the cyclophosphamide group, while mild and transient leukopenia occurred in another patient (WBCs count was 3.7×103/μL), who was having mild acute bronchitis at the time. In the levamisole group, none of the patients developed neutropenia but the leukocyte count was lower at the end of levamisole treatment than before the start of treatment. Such a reduction was significant in patients who were able to successfully stop steroids for more than two weeks (p=0.007), and non-significant in the remaining patients (p=0.63). Although none of the patients in the cyclophosphamide group received MESNA, hemorrhagic cystitis did not occur in any of them.
All patients were compliant with treatment and none of the patients discontinued therapy before the expected date. The overall side effects were mild and both drugs were well tolerated. However, in those patients who developed infection, hepatotoxicity, or leukopenia, the dose of cyclophosphamide was delayed for 1–2 weeks until recovery from these side effects. This occurred twice in 3 patients and once in 7 patients during the course of cyclophosphamide pulses.
Discussion
In our center, we have been using pulse cyclophosphamide since 1991 in certain cases of idiopathic nephrotic syndrome when oral cyclophosphamide therapy is indicated but they show non-compliance, are more vulnerable to side effects such as past history of recurrent infection episodes, or show both effects. Levamisole is also used in many of these patients as an alternative to cyclophosphamide.
On May 1998, we conducted this randomized prospective trial to study the efficacy and safety of levamisole versus pulse cyclophosphamide in steroid-dependent minimal change nephrotic syndrome children. We previously reported on the short-term results of levamisole therapy as well as on the results of cyclophosphamide pulse therapy [13, 14]. Here, we report on the long-term comparative outcome of this study. To our knowledge, this is the first study comparing both treatments in this group of patients.
At the commencement of the study, 37.5% of our MCNS children were hypertensive. This incidence is far above that reported by ISKDC (13%) in 1978 [15]. This big difference may be due to differences in the definition of hypertension (ISKDC defines it as diastolic blood pressure above the 98th percentile). It may also be attributed to steroid therapy in our patients (ISKDC report is concerned with patients at the time of diagnosis of their nephrotic syndrome). Sixty-five percent of children were at or above the 50th percentile of height. This may reflect our policy of turning to an every other day steroid as soon as remission has occurred in treatment of relapse.
High failure rate in both groups at six months may be partially explained by discontinuation of the steroids after the first two months and maintaining the patients on the adjunctive therapy only for a further four months. At short- and long-term levels, both levamisole and pulse cyclophosphamide were comparable in terms of their abilities to maintain remission. Fifteen percent of levamisole patients were in remission two years after completion of all medications, in contrast to only 5% in the cyclophosphamide group, and the steroid-free remission had a larger median in the former group. However, these differences were not statistically significant. Many other reports have documented good results of levamisole in nephrotic patients with significant reduction of relapse rate [16, 17, 18]. In the Ksiazek and Krynski study [16], 45.5% of steroid-dependent nephrotic patients could withdraw from steroids and maintain remission for more than six months after levamisole therapy.
In the two studies that compared oral and intravenous cyclophosphamide in children with steroid-dependent nephrotic syndrome [4, 19], the percentages of two-year pulse cyclophosphamide-induced remission were remarkably different (50% vs. 18.6%). However, their conclusions were similar and they were in favor of intravenous cyclophosphamide as a safe and effective therapeutic modality with lower cumulative dose in children with steroid-dependent nephrotic syndrome. Furthermore, Bircan and Kara [4] concluded that it is the drug of choice for this group of patients. In their study [4], the number of patients in remission for two years was significantly higher in the intravenous- than in the orally-treated group (50% versus 33.3%). However, the mean age, a factor that may affect the outcome, was lower in their intravenous-treated group compared to that in our study (3.5 vs. 7.38 years). Additionally, the cumulative steroid dose was significantly higher, as they continued steroids throughout the period of cyclophosphamide therapy. Furthermore, the protocol of steroid withdrawal after discontinuation of cyclophosphamide, the percentage of patients that became non-steroid-dependent, the previous adjunctive therapy and the renal pathology were not indicated in Bircan and Kara study. In the more recent study of Prasad et al [19], the actuarial cumulative sustained remission was almost identical in the I.V. cyclophosphamide (18.6%) and oral cyclophosphamide groups (19%) after two years of therapy. In our study, the two-year remission rate in the I.V. cyclophosphamide group was lower than that of the I.V. cyclophosphamide group in the Prasad et al study [19] (5% vs. 18.6%). It was even worse than the result from the oral cyclophosphamide group in the same study (19%), which is the lowest reported result obtained with the oral form in the literature. However, renal pathology may have an effect on the variability of the results. In the Prasad et al study [19], renal biopsy was only performed for 54% of patients; 36% of them had focal segmental glomerulosclerosis, while all our patients had biopsy-proven MCNS. Additionally, racial and genetic factors may alter the pattern of response to therapy so that the widely variable response rate to oral cyclophosphamide therapy seen in these patients reported in the literature (19%–75% at 1–3 years [19, 20, 21, 22, 23, 24, 25, 26, 27]) may be extrapolated to intravenous form. Furthermore, the conclusion of the previous two studies [4, 19], that the I.V. form is safe, was not tested over the long term.
On the other hand, in the only reported study comparing oral cyclophosphamide with levamisole in children with frequently relapsing steroid-dependent nephrotic syndrome [6], retrospective analysis of the data of 51 patients was performed. Twenty-four patients received levamisole 2.5 mg/kg on alternate days for at least 6 months, while 27 received oral cyclophosphamide 2–2.5 mg/kg for 8–12 weeks. Levamisole significantly reduced relapse rate, and cumulative steroid dose was significantly higher in the levamisole group. Nevertheless, this difference became statistically insignificant when analysis of covariance was performed to compensate for possible bias in patient selection. The authors concluded that levamisole might be considered an alternative for cyclophosphamide as a second-line agent with possibly less long-term side effects in this group of patients. They stated that the potential for serious side effects with cyclophosphamide was at times great enough for parents to prefer levamisole therapy. They were concerned about the cost of levamisole treatment. The cost/kg of one year of levamisole therapy was $30 versus only $0.50 for a cyclophosphamide eight-week course. The authors recommended a prospective study to verify their conclusions. In our prospective study, similar results were obtained when comparing levamisole with intravenous cyclophosphamide. Additionally, in our developing country, the ethical issue of using cyclophosphamide in nephrotic children is much more complicated. The parents are frequently illiterate and dependent on the treating physician. On discussing the possible side effects with them, they “authorize” the treating physician to decide the treatment. In contrast to western countries, the cost of levamisole in our country is much cheaper. The cost of a levamisole course for six months is only $0.29 versus $0.59 for a six-month intravenous cyclophosphamide course.
Abeyagunawardena et al [28] retrospectively analyzed the treatment modalities of 863 frequently relapsing steroid-dependent nephrotic children over 20 years. Among their patients, levamisole became the main steroid-sparing agent after publication of the British Association of Paediatric Nephrology guidelines for treatment of nephrotic syndrome in 1994. Levamisole was prescribed for 113 children, and in 65 of them it was the first prescribed steroid-sparing agent. Levamisole was effective in maintaining remission in 30% of patients when prescribed as the first steroid-sparing agent; almost half of them are currently off-treatment. Additionally, levamisole maintained remission in 66% of post-cyclophosphamide steroid-dependent patients.
In our study, the overall short-term side effects were mild. Both drugs were well tolerated and none of the patients discontinued therapy due to side effects, correlating with other reports involving the use of levamisole and pulse cyclophosphamide in nephrotic syndrome [4, 16, 17, 18, 19]. However, the possible long-term side effects of cyclophosphamide, including fertility issues and malignancy, remain to be tested.
In view of the comparable long-term results of levamisole and intravenous cyclophosphamide, our study indicates that levamisole may be safely tried for six months in children with steroid-dependent MCNS before adopting other types of adjunctive therapy that may cause serious side effects. Longer treatment periods may be expected to induce longer remission. Therefore, further studies are necessary to verify the effects of long-term levamisole therapy. However, the balance between the possible prolongation of the treatment period and the frequency of complications (not constant but unpredictable) should be considered. Additionally, a prospective study comparing levamisole, oral cyclophosphamide and intravenous cyclophosphamide in this group of patients may be of value.
References
Glassock RJ, Cohen AH, Adler SG (1996) Primary glomerular diseases. In: Brenner BM (ed) The kidney. WB Saunders, Philadelphia, PA, p 1392–1497
International Study of Kidney Disease in Children (1981) The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. J Pediatr 98:561–564
Trompeter RS (1986) Minimal change nephrotic syndrome and cyclophosphamide. Archiv Dis Child 16:727–729
Bircan Z, Kara B (2003) Intravenous cyclophosphamide is the drug of choice for steroid dependent nephrotic syndrome. Pediatr Int 45(1):65–67
British Association for Paediatric Nephrology (1991) Levamisole for corticosteroid-dependent nephrotic syndrome in childhood. Lancet 337:1555–1557
Alsaran K, Grisaru S, Stephens D, Arbus G (2001) Levamisole vs. cyclophosphamide for frequently-relapsing steroid-dependent nephrotic syndrome. Clin Nephrol 56(4):289–294
International Study of Kidney Disease in Children (1974) Prospective, controlled trial of cyclophosphamide therapy in children with the nephrotic syndrome. Lancet 2:423
Schwartz GJ, Brion LP, Spitzer A (1987) The use of plasma creatinine concentration in estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 34:571
Gandhi R, Thomas C (1990) Cyclophosphamide pulse therapy in frequently relapsing nephrotic syndrome. Nephron 55:444
Broyer M, Meyrier A, Naudet P, Habib R (1998) Minimal changes and focal segmental glomerular sclerosis. In: Davison AM, Cameron JS, Grünfeld JP, Kerr DNS, Ritz E, Wingearls CG (eds) Oxford textbook of clinical nephrology. Oxford University Press, Oxford, p 294–535
National High Blood Pressure Education Program Working Group (1996) Update on the 1987 task force report on high blood pressure in children and adolescents. Pediatrics 98:649–658
Kaplan DW, Mammel KA (1999) Adolescence. In: Hay JrWW, Hayward AR, Levin MJ, Sandheimer JM (eds) Current paediatric diagnosis and treatment. Appleton & Lang, Norwalk, CN, p 102–145
Donia AF, Amer GM, Ahmed HA, Gazareen SH, Moustafa FE, Shoeib AA, Ismail AM, Khamis S, Sobh MA (2002) Levamisole: adjunctive therapy in steroid dependent minimal change nephrotic children. Pediatr Nephrol 17:355–358
Donia AF, Gazareen SH, Ahmed HA, Moustafa FE, Shoeib AA, Ismail AM, Khamis S, Sobh MA (2003) Pulse cyclophosphamide inadequately suppresses reoccurrence of minimal change nephrotic syndrome in corticoid-dependent children. Nephrol Dial Transplant 18:2054–2058
International Study of Kidney Disease in Children (1978) Nephrotic syndrome in children. Prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. Kidney Int 13:159–165
Ksiazek J, Krynski J (1995) Evaluation of the efficacy of levamisole in corticosteroid-dependent nephrotic syndrome in children. Pediatr Pol 70(12):1037–1042
Fu LS, Chi CS (2000) Levamisole in steroid-sensitive nephrotic syndrome children with steroid-dependency and/or frequent relapses. Acta Paediatr 41(2):80–84
Bagga A, Sharma A, Srivastava RN (1997) Levamisole therapy in corticosteroid-dependent nephrotic syndrome. Pediatr Nephrol 11(4):415–417
Prasad N, Gulati S, Sharma RK, Singh U, Ahmed M (2004) Pulse cyclophosphamide therapy in steroid-dependent nephrotic syndrome. Pediatr Nephrol 19(5):494–498
Srivastava RN, Agarwal RK, Chowdhary VP, Moudgil A, Bhuyan UN, Sunderram KL (1987) Cyclophosphamide therapy in frequently relapsing nephrotic syndrome with and without steroid dependence. Int J Pediatr Nephrol 6:245–250
Shohet I, Meyerovitch J, Aladjem M, Boichis H (1988) Cyclophosphamide in treatment of minimal change nephrotic syndrome. Eur J Paediatr 147:239–241
Garin EH, Pryor ND, Fennell RS, Richard GA (1998) Pattern of response to prednisolone in idiopathic minimal lesion nephrotic syndrome as a criterion in selecting patients for cyclophosphamide therapy. Am J Dis Child 142:985–988
Arbeitsgemeinschaft fur Padiatrische Nephrologie (1987) Cyclophosphamide treatment of steroid dependent nephrotic syndrome: comparison of eight week with 12 week course. Arch Dis Child 62:1102–1106
Ueda N, Kuno K, Ito S (1990) Eight and twelve week courses of cyclophosphamide in nephrotic syndrome. Arch Dis Child 65:1147–1150
Kashtan C, Melvin T, Kim Y (1988) Long-term follow up of patients with steroid-dependent minimal change nephrotic syndrome. Clin Nephrol 29:79–85
Kemper M, Altrogge H, Ludwig K, Timmermann K, Müller-Wiefel D (2000) Unfavorable response to cyclophosphamide in steroid-dependent nephrotic syndrome. Pediatr Nephrol 14:772–775
Gulati S, Pokhariyal S, Sharma RK, Elhence R, Kher V, Pandey CM, Gupta A (2001) Pulse cyclophosphamide therapy in frequently relapsing nephrotic syndrome. Nephrol Dial Transplant 16(10):2013–2017
Abeyagunawardena AS, Dillon MJ, Rees L, van’t Hoff W, Trompeter RS (2003) The use of steroid-sparing agents in steroid-sensitive nephrotic syndrome. Pediatr Nephrol 18:919–924
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Donia, A.F., Ammar, H.M., El-Agroudy, A.EB. et al. Long-term results of two unconventional agents in steroid-dependent nephrotic children. Pediatr Nephrol 20, 1420–1425 (2005). https://doi.org/10.1007/s00467-005-1943-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-005-1943-4