Abstract
It is generally believed that infants are more susceptible to development of renal scarring after pyelonephritis than children over 5 years old. This view has led to differences in investigations and treatment according to age. The aim of this prospective study was to assess the occurrence of renal parenchymal lesion in children over 5 years admitted with a first-time symptomatic urinary tract infection (UTI). Between October 2000 and April 2002, 52 children aged over 5 years who were admitted to our department with probable acute pyelonephritis (APN) and a positive urine culture were included in this study. All children received antibiotics for 14 days. During the acute phase of infection, scintigraphy with technetium-99 m -labeled dimercaptosuccinic acid (DMSA) and ultrasonography (US) were done. Voiding cystourethrography (VCUG) was performed in all children early in the course of the illness, generally within 5–7 days of hospitalization. When scintigraphy showed renal parenchymal changes, repeat scintigraphy was done after at least 3 months to assess the progression of renal abnormalities. Of the 52 children with a first-time documented pyelonephritis, cortical scintigraphy showed renal lesion in 41 children (78.8%). US was normal in all children with normal renal scintigraphy, while it detected renal abnormalities in 16 of the 41 (39 %) with abnormal scintigraphy ( p <0.0001). Topographic analysis of the 165 focal lesions showed that 42.4% were localized to the upper poles, 17.5% to the middle third, and 40% to the lower poles of the kidneys. Repeat scintigraphy showed persistent lesions corresponding to those on the initial scan in nine (28.2%) of the 32 children. Renal lesions had partly regressed in 23 (71.8%) of the patients who underwent repeat scintigraphy. Vesicoureteral reflux was observed in 13.4% of kidneys and renal parenchymal abnormalities were identified in 71.4% and 72.2% of renal units, respectively, with and without reflux ( p >0.05). In conclusion, our data did not confirm the conventional opinion that the risk of renal scarring after pyelonephritis is low in children over the age of 5 years. Our findings suggest that renal scintigraphy may be a more appropriate method of investigation than VCUG for evaluation of the children over 5 years with acute pyelonephritis. Additionally, the frequency of scintigraphic changes is high, and a strategy based exclusively on ultrasound findings would miss about 61% of the abnormal renal units. We recommend that all children, irrespective of age, will benefit from further investigations that might prevent or limit the development of scarring process and renal complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is generally believed that very young children are more susceptible to development of renal scarring after pyelonephritis than patients over 5 years old. This view has led to differences in investigations and treatment according to age [1]. The children with renal involvement are at risk of permanent renal damage, which may lead to hypertension, proteinuria, hyposthenuria, complications during pregnancy, and even renal failure [2].
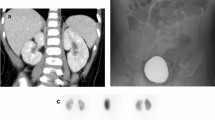
The diagnosis of urinary tract infection (UTI) in children is based on significant bacteriuria. The level of infection (i.e., whether the infection involves the kidneys or only the lower urinary tract) is of great importance for the choice of treatment and prognosis. Because the clinical symptoms are nonspecific, the diagnosis of renal infection needs to be supported by laboratory data and radiologic imaging. Renal cortical scintigraphy with technetium-99 m -labeled dimercaptosuccinic acid (DMSA), although not perfect, appears to be the best clinically applicable standard of reference for the diagnosis of acute pyelonephritis [3, 4, 5]. It is considered the most sensitive technique for the identification of the renal parenchymal change in acute pyelonephritis (APN), as well as in the detection of scarring. Acute DMSA renal scan defects persisted as renal scarring in 36–52% of kidneys [6]. The sites of new renal scarring corresponded to those sites of APN seen on initial DMSA renal scans, confirming the primary role of the acute inflammatory response to infection in the etiology of renal scarring [7].
Some controversy exists about the usefulness of acute renal scintigraphy. Many pediatric nephrologists share the view that the diagnosis of complicated UTI is based on clinical and biological data, and that scintigraphy is generally not necessary for the diagnosis [8, 9]. The goal of this prospective study was to assess the occurrence of renal parenchymal lesion in children over 5 years admitted with a first-time symptomatic UTI.
Patients and methods
Fifty-two patients (8 boys, 44 girls) aged 5–12 years (mean 7 years 6 months) were included in the study between October 2000 and April 2002. All children admitted to hospital because of high suspicion of APN, defined as follows: fever, with a temperature ≥38.5°C, ESR>20 mm/hr or increased C-reactive protein, alterations in the urinary sediment (leukocyturia, bacteriuria, hematuria) and a positive urine culture result (growth of single organism with colony counts equal to or greater than 105 colony-forming units/ml). Samples were collected as clean-voided midstream urine. Only those with proven UTI were included in the study. One hundred four kidneys were investigated by DMSA scan and renal ultrasound within the first days after admission. When scintigraphy revealed renal parenchymal lesions, a second scintigraphy was done at least 3 months later to evaluate the progression of renal lesions. The appearance of new lesions was recorded. Scintigraphy was done with intravenous injection of DMSA as described by Merrick et al. [10].
Images were obtained by means of a gamma equipped with a high-resolution collimator after an intravenous injection of Tc-99 m DMSA scintigraphy, according to a standard schedule [11]. About 3–4 hours after injection with the tracer, one posterior, one anterior and two posterior oblique images of the kidneys were acquired, with the patient prone below the camera. The fractional left and right renal activity was calculated for each kidney. The renal scintigraphic patterns were independently interpreted by two senior nuclear-medicine physicians, and the criteria used for the interpretation of the images did not change during the period of the investigation. A kidney with regular shape and a tracer uptake that appeared to be homogenous was considered as normal. Single or multiple cortical defects, focal or diffuse photopenic patterns in one kidney were considered as abnormal [12, 13]. The scan was considered to be abnormal for an old lesion (scar) when one or more areas of focal decreased uptake associated with contraction and loss of volume in the involved cortex were noted [8].
Patients with previous history of UTI, structural abnormalities such as neurogenic bladder, posterior urethral valves, ureteroceles or other congenital anomalies were excluded from the analysis. A kidney uptake of 45–55% of the total renal activity was considered as normal (symmetrical renal split function). The involvement of each kidney was visually graded as mild (focal defect in uptake), moderate (uptake of renal radionuclide of 20–40%) and severe (shrunken kidney with uptake less than 20%) [14]. We defined renal scars as persistent changes in the same location; complete or partial reversible lesions as complete or partial resolution of changes that had been observed on first scintigraphic examination; and new lesions not present during the acute phase of UTI. All scintigrams were independently assessed by two experienced radiologist who used standard criteria previously defined by Patel et al. [15].
Each lesion present in the late scan was directly compared by topographic analysis with the acute scan. A lesion present in the initial scan that persisted in the late scan was defined as a persistent lesion. A lesion present in the acute scan but no longer evident in the late scan was defined as a reversible lesion [3]. Renal ultrasound was performed on computerized sonographic units equipped with 3.5 MHz and 5.0 MHz transducers. The renal sonograms were interpreted by a pediatric radiologist without the knowledge of the findings from DMSA scans. The criteria of renal abnormality were: focal or generalized hyperechogenicity or hypoechogenicity, increase in renal size, loss of corticomedullary differentiation, thickened pelvic wall, irregular outlining of the kidney and parenchymal reduction. For all patients a VCUG was done early in the course of the illness, generally within 5–7 days of hospitalization and always before the patient was discharged from the hospital [16]. The radiographic cystograms were evaluated for the presence and grade of reflux using the international grading system [17].
Vesicoureteral reflux (VUR) was graded as mild, moderate, or severe by radionuclide technique [18]. The radionuclide cystography was graded as follows: Grade I reflux was defined as reflux into the ureter; grade II was reflux into a non-dilated collecting system; grade III was reflux into a mildly dilated collecting system; grade IV was reflux into a moderately dilated collecting system; and grade V was reflux into a severely dilated collecting system [19]. For the purpose of comparison between the two studies, radiographic and radionuclide cystography grades I and II correspond to mild, grade III to moderate, and grade IV and V to severe reflux. Patients were treated for 14 days with an antibiotic chosen according to bacterial susceptibility tests.
After the initial treatment, children with parenchymal lesions on the DMSA scan were placed on continuous prophylactic antibiotics until the second follow-up investigation, whether or not VUR was present. Patients with VUR were also kept on prophylaxis regimens for a period of at least 6 months. It was decided that all children enrolled in the study would receive this prophylaxis until the second scan, in order to avoid possible bias. No patient had a breakthrough infection during this period. Control urine cultures were taken in all children after treatment, 6 weeks, 3 months and 6 months, and more if clinically indicated.
No patient was excluded from the analysis during the hospital stay. Follow-up scan was performed if the initial scan was abnormal. Therefore, late scans were not performed in 11 patients, because of normal renal scan on the initial scintigraphy. Later, nine patients among the remaining 41 were lost to follow-up scan, and, because of the absence of a late renal cortical scintigraphy, they were not included in the analysis of renal scarring. Thus, 32 patients (64 renal units) with a positive acute DMSA scan remained eligible for analysis. The chi-squared procedure and the Fisher exact test were used to determine the statistical significance of the relationships between variables. A p value below 0.05 was considered statistically significant.
Results
Fifty-two patients, 44 females and 8 males, aged 5–12 years (mean 7.6 years) with a first documented UTI were enrolled in the study. The median time between the diagnosis of UTI and cortical scintigraphy was 3.5 days (range: 1–6 days). Of the 52 children with a first documented pyelonephritis, 41 (78.8%) had abnormal cortical scintigraphy(seven unilateral and 34 bilateral), and 11 (21.1%) had normal kidneys (Table 1). US was normal in all children with normal renal scintigraphy, while it detected renal abnormalities in 16 of the 41 (39%) with abnormal scintigraphy ( p <0.0001).
The number of kidneys involved in disease was 75 (38 left and 37 right). The extent of changes in DMSA scan was mild in 31/75 kidneys (41.3%), moderate in 29/75 kidneys(38.6%) and severe in 15/75 kidneys (20%).
Topographic analysis of the 165 focal lesions showed that 42.4% were localized to the upper poles,17.5% to the middle third ,and 40% to the lower poles of the kidneys. A voiding cystourethrogram was obtained for all children. VUR was documented in 14/104 kidneys (13.4%). Table 2 shows VCUG findings according to number of patients and renal units, respectively. The degree of reflux was mild (grades I and II) in 9/14 kidneys (64.2%), moderate (grade III) in 4/14 kidneys (28.5%) and severe (grades IV and V) in 1/14 kidneys (7.1%). As shown in Table 3, the frequency of renal parenchymal abnormalities in the presence of VUR and in non-refluxing renal units was similar (71.4% vs 72.2%, p >0.05).
Parenchymal abnormalities on scintigraphy were associated with mild reflux in 44.4% of the nine kidneys and moderate to severe reflux in 100% of the five renal units shown in Fig. 1. Although the rate of DMSA abnormality in kidneys with moderate to severe reflux were higher than those with mild reflux, the difference did not reach statistical significance ( p =0.086).
All these children were reassessed with a second renal evaluation after an average of 4 months. Repeat scintigraphy showed persistent lesions corresponding to those on the initial scan in 11 (29.6%) of the 64 renal units. Renal lesions had partially regressed in 45 (70.3%) of the renal units who underwent a follow-up scan. Residual lesions compared with the initial lesions are presented in Table 4. In all patients, persistent lesions in the late scan correspond exactly to the sites of acute pyelonephritis seen on the acute DMSA scans. No new abnormalities developed in previously normal contralateral kidney or in any other areas of the involved pyelonephritic kidney that were normal on the initial scan.
Discussion
The ultimate goal of treatment for pyelonephritis in children is prevention or reducing the morbidity and long-term clinical sequelae of renal scarring, including arterial hypertension, hyposthenuria, proteinuria and chronic renal failure [20, 21, 22, 23]. Experimental investigations have shown that inflammation has an important role in the pathogenesis of renal damage in pyelonephritis, which involves the local infiltration of polymorphonuclear leukocyte and the extracellular release of cytotoxic metabolites [24, 25, 26].
DMSA scintigraphy is currently considered the imaging agent of choice for estimating the presence and extension of acute parenchymal changes as well as the development of permanent renal scarring. It has been compared with histopathologic findings, and there has been 97% agreement between them [27]. DMSA uptake reflects renal tubular cell function and is therefore affected by both intrarenal blood flow and proximal tubular cell membrane transport [28]. Alteration of either or both of these parameters may result in focal or diffuse areas of diminished uptake. Experimental studies have reported focal ischemia associated with APN. Hill and Clark [29] found evidence of marked cortical vasoconstriction in areas of acute inflammation in rabbits with acute renal infection, with inflammatory cells obstructing the peritubular capillaries. Further ischemia-related alteration to renal tubular cell function results from the release of superoxide produced, in part during reperfusion of ischemic tissue [30]. Superoxide generates oxygen radicals that are toxic not only to the bacteria, but also to the surrounding tubular cells [31]. According to this hypothesis, the combination of interstitial damage from both toxic enzymes and ischemia that produces the inflammatory changes observed on DMSA renal scans during APN may be responsible for subsequent development of irreversible renal cortical scarring. In this study, both early and late DMSA scans were performed, since it was important to assess whether a normal renal unit on a late scan was also normal on the initial scan or was the consequence of a perfect healing process.
Experimental studies in piglets demonstrated that DMSA renal scan has sensitivity from 80–91% and specificity from 99–100% for detecting acute pyelonephritic lesions [1, 2, 32]. The undetected lesions by DMSA scan were attributed to grossly unapparent and microscopic clusters of inflammatory cells that involved less than 1% of the renal parenchyma [4]. It is also well known that DMSA scintigraphy reflects the function of proximal tubular cells and the intrarenal blood flow [28, 32]; consequently, an infection limited to the papilla and the medulla may not be clarified on a DMSA renal scan. This study revealed a high frequency of acute inflammatory changes on DMSA scan in 78.8% of children (72.1% of renal units), although the clinical and biological criteria were compatible with APN. This is similar to the findings reported by others [33, 34, 35, 36, 37, 38]. These data emphasize the fact that older children with first-time febrile UTI are at risk for acute inflammatory renal parenchymal damage.
VUR is a known risk factor for renal scarring. It has been found in up to 88% of children with DMSA scans during febrile UTI [8, 37, 38]. In our experience, VUR was found in 10/14 kidneys (71.4%) with evidence of pyelonephritis on DMSA scan. VUR was absent also in 65/90 kidneys (72.2%) with renal parenchymal involvement, which emphasizes that abnormalities at DMSA scan commonly occur in the absence of reflux [18, 39]. Reflux was present in 28.5% of normal kidneys without any abnormality. Our findings agree with the observations of other investigators. Biggi found acute pyelonephritis in 70 out of 101 children (69%) hospitalized with the diagnosis of an acute febrile UTI. VUR was present in 27% of the renal units with evidence of acute pyelonephritis on DMSA renal scan and absent in 47%.
In 80% of kidneys with abnormal DMSA scans, VUR of grades 4 to 5 was found. Reflux was present in 13% of kidneys with normal scan findings [8]. Majd found acute pyelonephritis in 62 out of 94 children with UTI (66%). Among the children with evidence of acute pyelonephritis on DMSA scan, VUR was present in 37% of children. DMSA scan findings of APN were present in 79% of children with VUR and 60% of those without demonstrable VUR. Reflux was present in 19% of renal units with normal DMSA scan [16].
Benador found acute pyelonephritis in 74 out of 111 children (67%) with UTI. VUR was presenting in 39% and absent in 61% [40]. Thus, our results confirm that a high percentage of febrile UTI, as documented by acute DMSA renal scan, can occur without the presence of a VUR. The higher incidence of renal parenchymal abnormalities in our patients seems contradictory to the general opinion that very young children are more susceptible to development of renal scarring after pyelonephritis than patients over 5 years old, and that scintigraphy is generally not necessary for the diagnosis [8, 9]. One theory for VUR as a cause of pyelonephritis concerns the elective localization of scars in the polar regions of the kidney [41, 42]. In the present study, the frequency of acute renal changes, as well as scars, was higher in upper and lower poles of the kidneys, whether there was a reflux or not. In contrast to findings by Benador et al. [43], these data are in accordance with the high percentage of polar abnormalities previously found. In some studies, DMSA changes remaining after 3 months from infection are considered to be persistent [11, 43]. In our experience, 32 out of 41 children with abnormal DMSA scan in the acute phase of the disease underwent a repeat scintigraphy between 3 months and 5 months after an index UTI. A persistent change was found in the 29.6% of the kidneys with an abnormal DMSA scan in the acute phase of disease. Similar results were reported by Biggi et al. [8].
Whether some of these cortical abnormalities on late DMSA scintigraphy were the consequence of the acute episode of infection or were preexisting (previously acquired pyelonephritic scars or congenital sequelae) cannot be determined. Cortical lesions that had partially regressed were found in 70.3% of kidneys, which confirms that the susceptibility of the renal parenchyma to infection in children over 5 years old is an important factor in the development of renal scarring. This finding agrees with the previous studies in children and adults, in which the development of new renal scars after a acute febrile pyelonephritis is common [40, 44, 45]. Age is generally considered an important risk factor for the development of renal sequelae. Previous studies using intravenous urography have shown that younger children are at greatest risk for renal scarring than older ones [46, 47]. Recent prospective studies using DMSA scintigraphy found no relationship between age at infection and developing renal damage [12, 33, 44, 48]. It has been suggested [49, 50] that the strategy for the use of DMSA renal scan should depend on age: “It should not be performed in children over 5 years with a first documented UTI and normal ultrasound.” Hoberman, in a recent prospective randomized study [51], included only children under 2 years of age, because some investigators have identified infants aged younger than 1 year as a group at particularly high risk for renal scarring [34]. In the present study, children over 5 years of age were included, based on the fact that other authors observed a high frequency of renal abnormalities in older children presenting with a first UTI [35, 44]. In this series, 25 out of 41 patients with abnormal DMSA scan were normal on US. This is in agreement with results in other studies and confirms that normal ultrasound findings do not accurately identify children with acute renal parenchyma involvement documented by DMSA scintigraphy [52].
Recently, it was reported that B-mode high-resolution ultrasonography performed by trained radiologists in patients with an average age of 2 years was as sensitive as the DMSA scan in detection of renal parenchyma changes in children with unobstructed pyelonephritis [53]. We conclude that a high percentage of children admitted with first-time febrile UTI have evidence of APN on DMSA scintigraphy. Most children with renal parenchymal involvement have no demonstrable VUR. Moreover, our results did not confirm the conventional opinion that the risk of renal scarring after pyelonephritis is low in children over the age of 5 years. Our findings suggest that renal scintigraphy may be a more appropriate method of investigation than VCUG for identifying children over 5 years at risk of renal sequelae. In addition, the frequency of scintigraphic changes is high, and a strategy based exclusively on ultrasound findings would miss a significant number of abnormal renal units. Accordingly, we recommend that all children hospitalized with UTI, irrespective of age, will benefit from further investigations that might prevent or limit the development of scarring process and renal complications.
References
Rickwood AM, Carty HM, McKendrick T, Williams MPL, Jackson M, Pilling DW , Sprigg A (1992) Current imaging of childhood urinary infections: prospective survey BMJ 304:663–665
Jacobson SH, Eklof O, Eriksson CG, Lins L-E, Tidgren B, Winberg J (1989) Development of hypertension and uremia after pyelonephritis in childhood: 27-year follow-up. BMJ 299:703-706
Parkhouse HF, Godley ML, Cooper J, Risdon RA, Ransley PG (1989) Renal imaging with 99 m Tc-labelled DMSA in the detection of acute pyelonephritis ; an experimental study in the pig. Nucl Med Commun 10:63–70
Rushton HG, Majd M, Chandra R, Yim D (1998) Evaluation of 99 m Tc dimercaptosuccinic acid renal scans in experimental acute pyelonephritis in piglets. J Urol 140:1169–1174
Pohl HG, Rushton HG, Park JS, Chandra R, Majd M (1999) Adjunctive oral corticosteroids reduce renal scarring: the piglet model of reflux and acute experimental pyelonephritis. J Urol 162:815–820
Bailey RR (1981) End-stage reflux nephropathy. Nephron 27:302–306
Rushton HG (1997) The evaluation of acute pyelonephritis and renal scarring with technetium 99 m -dimercaptosuccinic acid renal scintigraphy: evolving concepts and future directions. Pediatr Nephrol 11:108–120
Biggi A, Dardanelli L, Cussino P, Pomero G, Noello C, Serina O, Spada A, Camuzzini G (2001) Prognostic value of the acute DMSA scan in children with first urinary tract infection. Pediatr Nephrol 16:800–804
Goldraich NP, Ramos OL, Goldraich IH (1989) Urography versus DMSA scan in children with vesicoureteric reflux. Pediatr Nephrol 3:6–8
Merrick MV, Utlley WS, Wild SR (1993) The detection of pyelonephritic scarring in children by radioisotope imaging. Br J Radiol 53:544–556
Pediatric Task Group of the EANM (1990) A radiopharmaceuticals schedule for imaging pediatrics. Eur J Nucl Med 2:98–111
Jakobsson B, Berg U, Svensson L (1994 ) Renal scarring after acute pyelonephritis. Arch Dis Child 70:111–115
Marra G, Barbieri G, Agnola CAD, Caccamo MR, Castellani MJ (1994) Congenital renal damage associated with primary vesicoureteral reflux detected prenatally in male infants. J Pediatr 124:726–730
Polito C, La Manna A, Rambaldi PF, Nappi B, Mansi L, Di Toro R (2000) High incidence of a generally small kidney and primary vesicoureteral reflux. J Urol 164:479-482
Patel K, Charron M, Hoberman A, Brown ML, Rogers KD (1993) Intra and interobserver variability in interpretation of DMSA scans using a set of standardized criteria. Pediatr Radiol 23:506–509
Majd M, Rushton HG, Jantausch B, Wiederman BL (1991) Relationship among vesicoureteral reflux, P-fimbriated Escherichia coli, and acute pyelonephritis in children with febrile urinary tract infection. J Pediatr 119:578–585
International Reflux Committee. Writing Committee: Lebowitz RL, Olbing H, Parkkulainen KV, Smellie JM, Tamminen Mobius TE (1985) International system of radiographic grading of vesicoureteral reflux. Pediatr Radiol 15:105–109
Connolly LP, Treves ST, Zurakowski D, Bauer SB (1996) Natural history of vesicoureteral reflux in siblings. J Urol 156:1805–1807
Cooper JA (1998) Kidney infection in children: role of nuclear medicine. In: Freeman LM (ed) Nuclear medicine annual 1998. Lippincott-Raven, Philadelphia, pp 225–246
Wanner C, Luscher T, Groth H, Hauri D, Burger HR, Greminger P, Kuhlmann U, Siegenthaler W, Vetter W (1985) Unilateral parenchymatous kidney disease and hypertension: results of nephrectomy and medical treatment. Nephron 41:250–257
Gill DG, Mendes de Costa B, Cameron JS, Joseph MC, Ogg CS, Chantler C (1976) Analysis of 100 children with severe and persistent hypertension. Arch Dis Child 51:951–956
Still JL, Cottom D (1967) Severe hypertension in childhood. Arch Dis Child 42:34–39
Steinhardt GF (1985) Reflux nephropathy. J Urol 134:855–859
Glauser MP, Meylan P, Bille J (1987) The inflammatory response and tissue damage. Pediatr Nephrol 1:615–622
Monga M, Roberts JA (1995) The possible role of granulocyte elastase in renal damage from acute pyelonephritis. Pediatr Nephrol 9:583–586
Meylan PR, Markert M, Bille J, Glauser MP (1989) Relationship between neutrophil-mediated oxidative injury during acute experimental pyelonephritis and chronic renal scarring. Infect Immun 57:2196–2202
Sfakianakis GN, Sfakianaki ED (1988) Nuclear medicine in pediatric urology and nephrology. J Nucl Med 29:1287–1300
de Lange MJ, Piers DA, Kosterink JG, van Luijk WH, Meijer S, de Zeeuw D, van der Hem GK (1989) Renal handling of technetium-99 m DMSA: evidence for glomerular filtration and peritubular uptake. J Nucl Med 30:1219–1223
Hill GS, Clark RL (1972) A comparative angiographic, microangiographic, and histologic study of experimental pyelonephritis. Invest Radiol 7:33–47
McCord JM (1985) Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med 312:159–163
Roberts JA, Roth JK Jr, Domingue G, Lewis RW, Kaack B, Baskin G (1982) Immunology of pyelonephritis in the primate model. V. Effect of superoxide dismutase. J Urol 28:1394–1400
Risdon RA, Godley ML, Parkhouse HF, Gordon I, Ransley PG (1994) Renal pathology and the 99 m Tc-DMSA image during the evolution of the early pyelonephritic scar: an experimental study. J Urol 151:767–773
Stokland E, Hellstrom M, Jacobsson B, Jodal U, Sixt R (1996) Renal damage one year after first urinary tract infection: role of dimercaptosuccinic acid scintigraphy. J Pediatr 129:815–820
Vanderfaeillie A, Flamen P, Wilikens A, Desprechins B, Piepsz A (1998) Technetium-99 m -dimercaptosuccinic acid renal scintigraphy in children over 5 years of age. Pediatr Nephrol 12:295–297
Rushton HG, Majd M (1992) Dimercaptosuccinic acid renal scintigraphy for the evaluation of pyelonephritis and scarring: a review of experimental and clinical studies. J Urol 148:1726–1732
Jakobsson B, Svensson L (1997) Transient pyelonephritic changes on 99 m Technetium-dimercaptosuccinic acid scan for at least five months after infection. Acta Paediatr 86:803–807
Stokland E, Hellstrom M, Jacobsson B, Jodal U, Lundgren P, Sixt R (1996) Early 99 m Tc-dimercaptosuccinic acid (DMSA) scintigraphy in symptomatic first-time urinary tract infection. Acta Paediatr 85:430–436
Jakobsson B, Nolstedt L, Svensson L, Soderlundh S, Berg U(1993) 99 m Technetium-dimercaptosuccinic acid scan in the diagnosis of acute pyelonephritis in children: relation to clinical and radiological findings. Pediatr Nephrol 7:591–592
Ditchfield MR, De Campo JF, Cook DJ, Nolan TM, Powell HR, Sloane R, Grimwood K, Cahill S (1994) Vesicoureteral reflux: an accurate predictor of acute pyelonephritis in childhood urinary tract infection? Radiology 190:413–415
Benador D, Benador N, Slosman DO, Nussle D, Mermillod B, Girardin E (1994) Cortical scintigraphy in the evaluation of renal parenchymal changes in children with pyelonephritis. J Pediatr 124:17–20
Rolleston GL, Maling TM, Hodson CJ (1974) Intrarenal reflux and the scarred kidney. Arch Dis Child 49:531–539
Rose JS, Glassberg KI, Waterhouse K (1975) Intrarenal reflux and its relationship to renal scarring. J Urol 113:400–403
Verber IG, Meller ST (1989) Serial 99mTc-dimercaptosuccinic acid (DMSA) scans after urinary infections presenting before the age of 5 years. Arch Dis Child 64:1533–1537
Benador D, Benador N, Slosman D, Mermillod B, Girardin E (1997) Are younger children at highest risk of renal sequelae after pyelonephritis? Lancet 349:17–19
Fraser IR, Birch D, Fairley KF, John S, Lichtenstein M, Tress B, Kincaid-Smith PS (1995) A prospective study of cortical scarring in acute febrile pyelonephritis in adults: clinical and bacteriological characteristics. Clin Nephrol 43:159–164
Winberg J, Bollgren I, Kallenius G, Mollby R, Svenson SB (1982) Clinical pyelonephritis and focal renal scarring. A selected review of pathogenesis, prevention, and prognosis. Pediatr Clin North Am 29:801–814
Berg UB, Johansson SB (1983) Age as a main determinant of renal functional damage in urinary tract infection. Arch Dis Child 58: 963–969
Rushton HG, Majd M, Jantausch B, Wiedermann BL, Belman AB (1992) Renal scarring following reflux and nonreflux pyelonephritis in children: evaluation with Technetium 99 m -dimercaptosuccinic acid scintigraphy. J Urol 147:1327–1332
Gordon I (1990) Urinary tract infection in paediatrics: the role of diagnostic imaging. Br J Radiol 63:507–511
Gleeson FV, Gordon I (1991) Imaging in urinary tract infection. Arch Dis Child 66:1282–1283
Hoberman A, Wald ER, Hickey RW, Baskin M, Charron M, Majd M, Kearney DH, Reynolds EA, Ruley J, Janosky JE (1999) Oral versus initial intravenous therapy for urinary tract infections in young febrile children. Pediatrics 104:109–111
MacKenzie JR (1996) A review of renal scarring in children. Nucl Med Commun 17:176–190
Morin D, Veyrac C, Kotzki PO, Lopez C, Dalla Vale F, Durand MF, Astruc J, Dumas R (1999) Comparison of ultrasound and dimercaptosuccinic acid renal scintigraphy changes in acute pyelonephritis. Pediatr Nephrol 13:219–222
Acknowledgements
The authors gratefully acknowledge Dr. Mohammad Ali Rafiee and Dr. Alireza Rezaei, from the Nuclear Medicine section, and Dr. Javad Jannati and Dr. Mehrzad Mehdizadeh, from the Department of Radiology, for their cooperation with the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ataei, N., Madani, A., Habibi, R. et al. Evaluation of acute pyelonephritis with DMSA scans in children presenting after the age of 5 years. Pediatr Nephrol 20, 1439–1444 (2005). https://doi.org/10.1007/s00467-005-1925-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-005-1925-6