Abstract
Recent identification of the urate transporter in the kidney (URAT1, encoded by SLC22A12) led to the molecular elucidation of idiopathic renal hypouricemia, which is a predisposition toward exercise-induce acute renal failure. One Japanese patient with renal hypouricemia demonstrated compound heterozygous mutations of the URAT1 gene (Q297X and IVS2+1G>A). It was suggested that these two mutations are recurrent mutations of the URAT1 gene in a Japanese population. In addition, we expect the prevalence of renal hypouricemia, 0.23%, from the analysis of serum urate levels in 1,730 Japanese children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypouricemia has been recognized as a predisposition toward exercise-induced acute renal failure (ARF). Case reports of idiopathic renal hypouricemia (MIM 220150) with exercise-induced ARF have been increasing, especially in Japan [1, 2]. The patients complain of severe loin pain with nausea and vomiting several hours after exercise [1, 2]. Although some patients required hemodialysis during their ARF courses, the short-term prognosis of these patients seems good. ARF demonstrates acute tubular necrosis on histological findings and multiple wedge-shaped areas of contrast enhancement on renal computed tomography scans after contrast medium administration [1, 2]. The patients demonstrated blood urate levels of 0.4–0.8 mg/dl (normal 2.8–7.9 mg/dl) after recovery from ARF [1, 2]. Recent identification of the urate transporter in the kidney (URAT1, encoded by SLC22A12) led to the molecular elucidation of idiopathic renal hypouricemia, and several URAT1 genetic mutations have been identified in patients with hypouricemia [3]. In this report two mutations of the URAT1 gene, which were identified from a Japanese boy with hypouricemia, are suggested to be recurrent mutations in the Japanese population. In addition, we describe the prevalence of renal hypouricemia estimated by determining the blood urate levels of 1,730 Japanese children.
Subjects and methods
The patient was a healthy 14-year-old boy, born from healthy parents, with mild obesity. He had no family history of acute renal failure or any renal diseases. A low level of uric acid was incidentally detected in blood serum, and he was admitted to our hospital for the analysis of renal function. Laboratory data of this patient showed the following: total protein, 7.5 g/l; blood urea nitrogen, 13.9 mg/dl; urate, 0.7 mg/dl; creatinine, 0.6 mg/dl; Ca, 9.7 mg/dl; iP, 2.9 mg/dl; Na, 139 mEq/l; K, 4.4 mEq/l; Cl, 101 mEq/l; HCO3 25.7 mmol/l. On urinalysis glucosuria or aminoaciduria was not observed, and there were no other abnormal data detected, but fractional excretion of uric acid was 0.74 (normal 0.04–0.14), suggesting a diagnosis of renal hypouricemia, not a dysfunction of the proximal renal tubule resulting in excessive urinary losses of amino acids, glucose, bicarbonate, phosphate, and uric acid. Tests were performed with pyrazinamide and probenecid, which are known inhibitors of the renal urate transporting system, to investigate uric acid cycling in the kidney. Fractional excretion of uric acid did not change after 3 g pyrazinamide or 2 g probenecid administration. Genomic DNA was extracted from peripheral blood lymphocytes. Polymerase chain reaction amplification from exon 1 to exon 10 of the URAT1 gene was performed with ten primer pairs corresponding to each exon. The cycling conditions were 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s for 30 cycles, followed by a final extension step at 72°C for 7 min. Both forward and reverse strands were directly sequenced using an automatic DNA sequencer (ABI Prism 310 DNA sequencer; PE Applied Biosystems, Foster City, Calif., USA). We could not analyze the URAT1 genes from the parents.
To determine the prevalence of hypouricemia in the Japanese population we investigated the serum urate levels of Japanese healthy children. This was a cross-sectional study performed in children aged 9–15 years living in Akita Prefecture, Japan, in 2003 (Akita Study 2003). A total of 1,730 children, of whom 923 were male and 807 female, were selected for sampling. No subject in this study had any chronic diseases or any medications. Blood sera taken from the children were stored at −20°C until the day of the study, and the serum urate levels of the subjects were determined. Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were also determined at the same time. A written consent form was obtained from the children’s parents. Statistical analysis was performed with the Mann-Whitney test.
Results
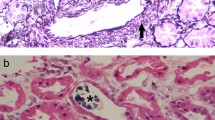
Two different URAT1 gene mutations were identified in the patient. One of the URAT1 alleles in this patient contained a C to T transversion in exon 5 which changed the glutamine codon (CAG) to a stop codon (TAG) at URAT1 codon 297 (Q297X) (Fig. 1a). The other URAT1 allele had a mutation (IVS2+1G>A) at the consensus donor splice site of intron 2 (Fig. 1b). It was not possible to analyze transcripts from URAT1 mRNA of the patient because the URAT1 gene is strictly expressed only in the kidney. However, this splice site mutation is expected to cause a splicing error of URAT1 mRNA, leading to severe dysfunction of the protein translated from the mutated transcript. Thus these two mutations cause an extremely low level of plasma uric acid through dysfunction of the urate transporter system in the kidney.
Molecular lesion in a patient with renal hypouricemia. a One of the URAT1 alleles contained a C-to-T transversion in exon 5 which changed the glutamine codon (CAG) to a stop codon (TAG) at URAT1 codon 297 (Q297X). b The other URAT1 allele had a mutation (IVS2+1G>A) at the consensus donor splice site of intron 2
The serum urate levels of male and female subjects were determined as 5.32±1.3 mg/dl (range 0.6–9.6) and 4.3±0.86 mg/dl (range 0.2–7.7), respectively. Mean serum urate concentrations and number for each year of age are shown in Fig. 2. Between the ages of 11 and 15 years boys had significantly higher means than girls. Four subjects, two boys and two girls, were diagnosed as having hypouricemia (serum urate levels of less than or equal to 2.0 mg/dl), with the serum urate levels of 0.6 mg/dl, 1.9 mg/dl, 0.2 mg/dl, and 1.9 mg/dl, respectively. The serum levels of AST and ALT were within normal limits in the four subjects, suggesting that the hypouricemia was not a complication of Wilson disease. Based on these results the prevalence of hypouricemia was estimated as 0.23% (4/1,730) in the Japanese population.
Discussion
To date there have been four reports of URAT1 gene mutations causing renal hypouricemia [3, 4, 5, 6]. The original study reporting molecular cloning of the URAT1 gene described three mutations (W258X, T217 M, E298D) as homozygous in three different patients each [3]. In the second study the URAT1 gene was sequenced in 32 unrelated idiopathic renal hypouricemia patients [4]. In this analysis URAT1 gene mutations were identified in 54 of the total 64 alleles, and the W258X mutation was recognized in 40 of 54 alleles, meaning the allele frequency of 74.1%. The R90H and the V138 M were identified in five alleles (allele frequency 9.3%) and two alleles (allele frequency 3.7%), respectively. In the third report six of seven patients with renal hypouricemia possessed mutations in the URAT1 gene, and five patients showed the W258X as a homozygous mutation, suggesting that W258X in the URAT1 gene is the predominant cause of renal hypouricemia in Japan [5]. In the most recent report the mutations and polymorphisms of the URAT1 gene were characterized in the general population, 1,875 consecutive subjects, in Japan [6]. The allele frequency of the W258X mutation was determined as 2.37% in the population. This frequency was unexpectedly high, and the mutation does not seem to be harmful in the general population. In this report the effects of 16 common polymorphisms of the URAT1 gene were also investigated, but none of those polymorphisms affected serum uric acid levels.
In the present report our patient had compound heterozygous mutations of Q297X and IVS2+1G>A. These two mutations were also reported as one allelic mutation of compound heterozygous mutations including the W258X in the second report [4]. However, there was no relationship between our case and the patient of the second report. Therefore the Q297X and the IVS2+1G>A may be recurrent mutations in the Japanese population.
In this report the prevalence of hypouricemia was estimated as 0.23% in the Japanese population. There are two previous reports describing the prevalence of hypouricemia [7, 8]. One study in the United State analyzed 5,000 patients and found 36 to have a serum uric acid level of less than 2.0 mg/dl on one or more occasions, indicating that the prevalence of hypouricemia was 0.72% [7]. The positive patients were clinically analyzed in further detail and only 8 of the 36 patients were in good health or not complicated. In the other report, from Japan, serum urate levels of 3,258 consecutive outpatients were measured at one hospital [8]. Two groups of hypouricemia were demonstrated in that study. Transient hypouricemia was defined as a serum urate level of 2 mg/dl or less on one or two urate determinations from the same patient. Persistent hypouricemia was defined as a serum urate value of 2 mg/dl or less on three separate measurements from the same patient. The results showed that eight patients (0.25%) had transient hypouricemia due to known causes, including diabetes mellitus, liver cirrhosis, hepatic cancer, allopurinol, and uricosuric drugs, and that five patients (0.15%) had persistent hypouricemia. The five with persistent hypouricemia had increased urate clearance in the renal clearance tests of urate and creatinine, indicating that they all had renal hypouricemia. The authors suggested that persistent hypouricemia is caused by a variety of isolated renal tubular secretory defects, not by other disorders. On our analysis the estimated prevalence may indicate the frequency of persistent hypouricemia, equivalent to renal hypouricemia, because all subjects were healthy, not complicated, and not medicated children. According to the four reports regarding the URAT1 gene mutations described above, the URAT1 gene mutations dominate the genetic etiology of renal hypouricemia [3, 4, 5, 6]. Therefore we can expect that the frequency of renal hypouricemia caused by URAT1 gene mutations may be close to the prevalence of renal hypouricemia estimated in this report.
References
Ohta T, Sakano T, Ogawa T, Kato J, Awaya Y, Kihara H, Kinoshita Y (2002) Exercise-induced acute renal failure with renal hypouricemia: a case report and a review of the literature. Clin Nephrol 58:313–316
Ishikawa I (2002) Acute renal failure with severe loin pain and patchy renal ischemia after anaerobic exercise in patients with or without renal hypouricemia. Nephron 91:559–570
Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, Hosoyamada M, Takeda M, Sekine T, Igarashi T, Matsuo H, Kikuchi Y, Oda T, Ichida K, Hosoya T, Shimokawa K, Niwa T, Kanai Y, Endou H (2002) Molecular identification of a renal urate-anion exchanger that regulates blood urate levels. Nature 417:447–452
Ichida K, Hosoyamada M, Hisatome I, Enomoto A, Hikita M, Endou H, Hosoya T (2004) Clinical and molecular analysis of patients with renal hypouricemia in Japan-influence of URAT1 gene on urinary urate excretion. J Am Soc Nephrol 15:164–173
Komoda F, Sekine T, Inatomi J, Enomoto A, Endou H, Ota T, Matsuyama T, Ogata T, Ikeda M, Awazu M, Muroya K, Kamimaki I, Igarashi T (2004) The W258X mutation in SLC22A12 is the predominant cause of Japanese renal hypouricemia. Pediatr Nephrol 19:728–733
Iwai N, Mino Y, Hosoyamada M, Tago N, Kokubo Y, Endou H (2004) A high prevalence of renal hypouricemia caused by inactive SLC22A12 in Japanese. Kidney Int 66:935–994
Van Peenen HJ (1973) Causes of hypouricemia. Ann Intern Med 78:977–978
Hisatome I, Ogino K, Kotake H, Ishiko R, Saito M, Hasegawa J, Mashiba H, Nakamoto S (1989) Cause of persistent hypouricemia in outpatients. Nephron 51:13–16
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takahashi, T., Tsuchida, S., Oyamada, T. et al. Recurrent URAT1 gene mutations and prevalence of renal hypouricemia in Japanese. Pediatr Nephrol 20, 576–578 (2005). https://doi.org/10.1007/s00467-005-1830-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-005-1830-z