Abstract
The newborn rat kidney is not fully developed until approximately 12 days after birth. Several lines of evidence suggest that angiotensin II (AII) participates in the postnatal development of the kidney. The aim of the present study was to analyze proliferating cell nuclear antigen (PCNA), fibronectin, α-smooth muscle-actin (α-SM-actin), and AII expression in renal cortex during development in rats born to mothers that received a normal (control) or increased (experimental) sodium intake during pregnancy. Ninety Wistar rats aged 1, 7, 15, and 30 days from the control and experimental groups were killed and the kidneys removed for histological and immunohistochemical studies. The results showed higher fibronectin, α-SM-actin, PCNA, and AII expression in the glomerular and tubulointerstitial areas of the renal cortex of 1- and 7-day-old animals, which decreased with renal development. The animals from the experimental group showed at 1 day of age a decrease in α-SM-actin, fibronectin, PCNA, and AII expression compared with controls of the same age ( P <0.05). In conclusion, our data show that increased sodium intake during pregnancy induces a reduction of α-SM-actin, fibronectin, and PCNA expression in the renal cortex tubulointerstitium and glomeruli of neonatal rats. These alterations may be related to the decrease of AII expression also observed in the renal cortex from these animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The nephrogenesis of rats begins on embryonic day 12 and is completed between 10 and 15 days after birth [1, 2]. Formation of the extracellular matrix (ECM) represents a key event in kidney cell differentiation [3, 4]. The principal components of the ECM are type IV and III collagen, fibronectin, laminins, and proteoglycans. Fibronectin is a glycoprotein that can interact with some proteins from cell or ECM, inducing changes in cellular migration and adhesion [4]. AII can stimulate the production of several ECM components by mesangial cells in culture [5].
Several lines of evidence show that the renin-angiotensin system (RAS) participates in renal development [6, 7, 8, 9, 10]. AII can act as a growth modulator of several cell types and tissues [6, 7]. Angiotensinogen expression in the rat kidney increases during late gestation and peaks during the newborn period, when it reaches considerably higher levels than in the adult. In the rat kidney, renin mRNA is detected from embryonic day 17 and continues to be high during late gestation [8]. Renin mRNA levels are approximately 20-fold and 10-fold higher, respectively, at 20 days of embryonic life and in newborns than in adults [9]. The renal AII content is several-fold higher in newborn rats and mice than in adults. There is also increased expression of AII receptors in neonatal rats [10].
The mRNA for type 1 AII receptor (AT1) is detected in the renal glomeruli of newborn rats during cellular proliferation and differentiation [11]. Increased type II AII receptor (AT2) was observed in the kidney during fetal life, followed by a decrease after birth, suggesting that AII can participate in cell differentiation [12]. It was recently demonstrated that gene expression for AT1 receptors (a subtype predominant in the kidney) is up-regulated after decreased sodium intake, suggesting that the expression of this receptor type is related to water and salt homeostasis [13].
The aim of the present study was to analyze cell phenotype modification and proliferation and ECM production during renal development in newborn rats from mothers that received a normal or increased sodium intake during pregnancy, and to assess the possible relationship between AII expression and immunohistochemical alterations in renal cortex. We also evaluated systolic blood pressure and renal function in 30-day-old rats.
Materials and methods
Animals and experimental protocol
Ninety female Wistar rats aged 1, 7, 15, and 30 days, born to 30 pregnant females weighing approximately 180 g, were used in this study. All rats were housed in rooms with controlled temperature about 25°C and a 12-h light/dark cycle. Food and water were supplied ad libitum. Pregnant females were carefully observed at the end of gestation to determine the exact birth date of the pups. During gestation and breast-feeding, 8 females received a normal sodium intake and 12 females received a 0.15 mol/l sodium chloride solution instead of water. After birth, pups were divided into the following groups: group 1 (control) rats aged 1 ( n =7), 7 ( n =7), 15 ( n =8), and 30 days ( n =16) from mothers that received a normal sodium intake and group 2 (experimental) rats aged 1 ( n =10), 7 ( n =10), 15 ( n =9), and 30 days ( n =23) from mothers that had an increased sodium intake. Newborn rats of each group were anesthetized with sulfuric ether and one kidney was removed and fixed in Boüin solution for 4 h and the other kidney was fixed in methacarn for 24 h. After fixation, the kidneys were rinsed in 70% ethanol and embedded in paraffin. Sections (3-μm) of renal tissue were used for histological and immunohistochemical studies. All experimental procedures were conducted in accordance with our institutional guidelines.
Urine and plasma samples were collected from pregnant females at the beginning and end of pregnancy using metabolic cages and 24-h urine volume was measured. The samples were frozen for subsequent analysis of osmolality, sodium, and potassium. Liquid and food consumption and body weight variation during pregnancy were measured in mothers of both groups. The blood pressure of pregnant rats was measured at the beginning and at the end of pregnancy by the tail cuff method.
Histological and immunohistochemical analysis
Sections (3-μm) of renal tissue from these animals were stained with Masson’s trichrome for histological analysis. The number of glomeruli of 30-day-old animals from the control and experimental groups was determined per area of renal cortex measuring 0.245 mm2. To analyze the planar glomerular area in renal sections, the outer edges of all glomerular tufts of each kidney were traced on a video screen, and the encircled areas were determined by computerized morphometry (Kontron Electronic System KS 300, Eching, Germany) [14].
The sections were incubated with the following antibodies for immunohistochemical studies: anti-α-SM-actin (Dako, Glostrup, Denmark), anti-fibronectin (Chemicon International, Temecula, Calif., USA), anti-AII (Peninsula Laboratories, San Carlos, USA) overnight at 4°C, and anti-proliferating cell nuclear antigen (PCNA) (Sigma, St. Louis, Mo., USA) for 30 min at room temperature. The reaction product was detected with an avidin-biotin-peroxidase complex (Vector Laboratories, Burlingame, Calif., USA). The material was counterstained with methylgreen, dehydrated, and mounted. Nonspecific protein binding was blocked by incubation with 20% goat serum in phosphate-buffered saline (PBS) for 20 min. Negative controls consisted of replacement of primary antibody with equivalent concentrations of normal rabbit IgG or mouse IgG for polyclonal and monoclonal antibodies, respectively. For evaluation of immunoperoxidase staining for fibronectin and α-SM-actin each grid field was graded semi-quantitatively and the mean score per kidney was calculated [14]. Each score reflected mainly changes in the extent, rather than the intensity, of staining and depended on the percentage of grid field showing positive staining: 0=absent or less than 5%; I=5%–25%; II=25%–50%; III=50%–75%; IV >75%. The semi-quantitative scoring system used to evaluate the immunohistochemical reaction is not only reproducible among different observers, but the results are highly correlated with those obtained by computerized morphometry [14, 15]. To obtain the numbers of AII- and PCNA-positive cells in the glomeruli and AII-positive cells in the cortical tubulointerstitium, all glomeruli and 30 grid fields from the renal cortical tubulointerstitium measuring 0.245 mm2 were evaluated, and the mean counts per kidney were calculated.
Analysis of AT1 receptor expression
Whole cortical tissue from one kidney of 8 animals of each group aged 1 day was homogenized in 2 ml of Triton X-100 lysis buffer [50 mM TRIS-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1 µg/ml aprotinin, 1 µg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride] at 4°C. After incubation for 5 min, lysates were centrifuged at 4°C for 15 min at 10,000 g. The AT1 receptor in these samples was evaluated by Western blotting [16]. Renal protein was loaded at 100 μg per well and separated on a 8% SDS-PAGE gel. Protein estimations were performed using the Hartree method [17]. Gels were eletroblotted onto a nitrocellulose membrane, incubated for 4 h in 30 ml of blocking buffer (PBS, 5% skimmed milk), washed in buffer (PBS, 0.1% Tween 20, pH 7.6), and incubated with anti-AT1 (1/100) (Santa Cruz Biotechnology, Santa Cruz, Calif., USA) in 5% bovine serum albumin (BSA) overnight at 4°C. Blots were washed and incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (Dako) (1/2,000) for 1 h at room temperature. Membranes were washed and the membrane-bound antibody detected by Supersignal West Pico Chemiluminescent Substrate (Pierce) and captured on X-ray film. Densitometry analysis was performed using the Image Quant computerized program.
Renal function studies
Renal function was evaluated in 15 30-day-old animals born of mothers that received a higher salt intake during pregnancy and in 10 animals of the same age born of mothers that received a normal sodium intake. The animals were anesthetized with an intraperitoneal injection of 170 mg/100 g urethane. The temperature was maintained at 37°C during the experiment. The femoral artery and vein were cannulated to collect blood samples and to inject fluids and the urinary bladder was cannulated to collect urine. The animals received a priming inulin dose of 12 mg/100 g followed by a maintenance dose of 42 mg/100 g per hour. After stabilization for about 60 min, urine was collected for a period of 90 min and blood was sampled at 45 and 90 min. Plasma and urine inulin was measured using the method of Fuehr et al. [18].
Statistical analysis
Data were submitted to analysis of variance with multiple comparisons by the Tukey test, with the level of significance set at P <0.05 for comparisons between analyzed parameters of the same group at different ages and Student′s t -test with Welch’s comparison for analysis of parameters between studied groups of the same age.
Results
Urinary volume, blood pressure, body weight variation, food consumption, and fluid intake
Urine volume (24-h) and liquid intake was higher in pregnant females that received a high salt intake compared with pregnant females from the control group. The body weight was also higher in pregnant females from the experimental group compared with pregnant females from the control group. These results reflect an increase of extracellular fluid volume in mothers from the experimental group. The systolic blood pressure of pregnant females was measured at the beginning and end of pregnancy and was not different between both groups (Table 1).
Plasma levels of sodium and potassium and plasma osmolality were not different between pregnant females from the control and experimental groups. Food consumption was not different between mothers from the control and experimental groups (Table 2).
Histological and morphometric analysis
Histological analysis showed glomeruli in different stages of evolution in renal cortex from 1- and 7-day-old animals, while 15- and 30-day-old animals had glomeruli in the final stage of differentiation (Fig. 1A and B). There were no changes in the number of glomeruli per renal cortex area measuring 0.245 mm2 in 30-day-old animals from the experimental group (4.09±0.21) compared with the control group (3.98±0.14) of the same age. Morphometric analysis did not show any alterations in the glomerular area of 30-day-old animals from the experimental group (3,772 μm2±116) compared with the control animals of the same age (3,757 μm2±117).
Immunohistochemical analysis
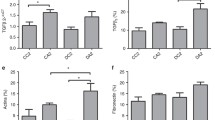
Staining for α-SM-actin in the glomerular and tubulointerstitial compartments of control rats was higher in 1-day-old animals compared with 30-day-old animals (Tables 3 and 4, Fig. 2C, D). The immunostaining for α-SM-actin was limited to arterial smooth muscle cells in the renal cortex of 30-day-old animals (Fig. 2D). One-day-old animals whose mothers received a high salt intake during pregnancy showed a tendency to a decrease in glomerular α-SM-actin expression (1.40±0.08) compared with control animals of the same age (1.67±0.12) (Table 4). In the tubulointerstitial compartment (Table 3) there was a significant reduction in α-SM-actin expression in 1-day-old animals from the experimental group (1.59±0.19) compared with 1-day-old animals from the control group (2.22±0.16) ( P <0.05).
Immunolocalization of proliferating cell nuclear antigen (PCNA) ( A and B) and α-SM-actin ( C and D) in renal cortex from 1- ( A and C) and 30-day-old rats ( B and D) from mothers that received a normal sodium intake (×280). Note that the number of PCNA-positive cells in the tubulointerstitial and glomerular compartments is higher in A than in B and that the immunostaining for α-SM-actin is more intense in C than in D
In control animals, glomerular fibronectin expression was higher in 1-day-old rats than in 30-day-old rats (Table 4, Fig. 1C, D). A decrease in glomerular fibronectin expression was observed in 1-day-old experimental animals (2.1±0.12) compared with the control animals of the same age (2.53±0.06) ( P <0.05).
The number of PCNA-positive cells per glomerulus was higher in 1- and 7-day-old control animals than in 30-day-old animals (Table 4, Fig. 2A, B). One-day-old animals from the experimental group (Table 4) also had a decrease in the number of PCNA-positive cells (4.99±0.59) compared with control animals of the same age (7.85±0.29) ( P <0.05).
The number of AII-positive cells was higher in the glomerular and tubulointerstitial compartments of the renal cortex of 1- and 7-day-old animals (Tables 3 and 4, Fig. 3A–C), while in 30-day-old animals the AII-positive cells were limited to the juxtaglomerular apparatus (Fig. 3C). The 1- and 7-day-old animals from the experimental group had a lower number of AII-positive cells per glomerulus (0.39±0.07, 0.58±0.08, respectively) compared with controls aged 1 (0.80±0.080) and 7 days (1.07±0.21) ( P <0.05) (Table 4). One-day-old animals whose mothers received a higher salt intake during pregnancy also had a reduced number of AII-positive cells in the cortical tubulointerstitial compartment (4.27 cells/0.245 mm2±0.42) compared with controls of the same age (5.87 cells/0.245 mm2±0.35) ( P <0.05) (Table 3).
AT-1 receptor analysis
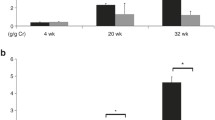
Our data show that there was a decrease in AT-1 receptor in renal cortex from the 1-day-old animals of the experimental group ( P <0.05) (Fig. 4).
AT1 protein expression in renal cortex of 1-day-old rat kidneys from mothers that received a normal sodium intake (bands 1–3) or increased sodium intake (bands 4–7) during pregnancy. Density analysis of 50-kDa bands showed a significant decrease in the level of AT1 in renal cortex from animals of the experimental group. * P <0.05 versus control of the same age
Renal function
Thirty-day-old animals whose mothers received an increased salt intake during pregnancy showed a decrease in glomerular filtration rate (GFR) (0.67 ml/min per 100 g±0.05) compared with controls of the same age (0.80 ml/min per 100 g±0.07) ( P <0.05) (Table 5).
Discussion
Our data showed increased α-SM-actin expression in the cortical, tubulointerstitial, and glomerular compartments from 1- and 7-day-old animals, followed by a decrease during renal development. These results show that mesangial cell and interstitial fibroblast activation occurs during kidney differentiation, as confirmed by the increased expression of fibronectin and PCNA in the renal cortex from 1- and 7-day-old animals. The activation of these cells may be at least in part due to an increase in renal AII production. Rats born from mothers that received a high salt intake presented at 1 day of age with a decrease of α-SM-actin, fibronectin, and PCNA expression in the renal cortex, as well a reduction in the number of AII-positive cells in glomerular and interstitial compartments compared with controls of the same age.
The α-SM-actin isoform is normally expressed by vascular smooth muscle cells, but interstitial fibroblasts and mesangial and tubular cells can also express this protein in a variety of renal diseases [19, 20, 21]. There is some experimental evidence suggesting that AII can induce this phenotypic modification of these cells [22, 23]. Chen et al. [23] reported that neonatal losartan treatment suppressed renal expression of α-SM-actin. The up-regulation of α-SM-actin synthesis by these cells is frequently associated with increased cell proliferation and ECM production [19, 24]. It has already been shown that human and rat fetal mesangial cells express α-SM-actin [25, 26] and that human fetal mesangial cells change their expression of this protein in the course of glomerular development [26]. During kidney development, a large-scale proliferation has been observed [27]. Therefore, changes in the renal expression of AII in animals born to mothers that received an increased sodium intake might affect renal cell proliferation and ECM production during postnatal development. Our results confirm this hypothesis, i.e., the increased sodium intake during the pregnancy provoked reductions of α-SM-actin, fibronectin, and PCNA in the renal cortex of neonatal rats. These alterations were related to a decrease in AII expression in the tubulointerstitial and glomerular compartments of the renal cortex. There was a temporal association suggesting a possible relationship between these factors. It has also been demonstrated that AII has important effects on cell growth and ECM production [23, 28]. Angiotensin converting enzyme (ACE) inhibition reduced cell proliferation in medullary tubules in postnatal rats [29] and treatment of neonates with AT1 antagonists suppresses the renal expression of α-SM-actin [23].
It has been shown that AII can act as a growth modulator of several cell types and tissues and can stimulate the production of several components of ECM by mesangial cells in culture [5]. McCausland et al. [29] found that angiotensin enzyme inhibition in postnatal rat results in decreased cell proliferation in the renal outer medulla. Blocking the AT1 receptor led to abnormal cell-matrix interactions and matrix assembly during postnatal development [23]. The RAS activity is increased in neonatal rats and the activity of plasma renin and ACE increases during gestation and is higher in neonates than in adult rats [30]. The expression of AII receptors is also higher in neonatal rats [10].
Our results also showed that 30-day-old rats born to mothers that received a higher sodium intake had higher systolic blood pressures than controls. A decline in GFR was also observed in 30-day-old animals from this group. These data demonstrate that disturbances occurring during intrauterine life can induce persistent alterations in the biology of the pups in adult life. It has previously been reported that a high-salt prenatal diet increases blood pressure and salt retention in spontaneously hypertensive rats [31]. Silva et al. [32] and Hazon et al. [33] found that adult offspring from high-salt dams had higher blood pressure, an increased renal AII content, and an absence of plasma renin changes in responses to higher salt consumption [32]. We also observed a decrease in expression of AT-1 receptors in 1-day-old rats born to mothers that received a higher salt intake compared with controls of same age. Since kidney development is influenced by the RAS, possible functional changes in the RAS may occur in offspring due to the lower AII levels in response to a high-salt intake of dams during pregnancy. The higher blood pressure observed in 30-day animals of the experimental group may be consequence of this.
In conclusion, our data show that an increased sodium intake during pregnancy induces reductions of α-SM-actin, fibronectin, PCNA, and AII expression in the renal cortex of neonatal rats, interfering with renal development and renal function during adult life. There was a temporal association between the decrease of AII expression and the reduction of α-SM-actin, PCNA, and fibronectin expression in renal cortex, suggesting a possible relationship between these findings.
References
Nigam SK, Aperia AC, Brenner BM (1996) Development and maturation of the kidney. In: Brenner BM, Rector FC (eds): The kidney. Saunders, Philadelphia, pp 72–98
Reeves W, Caulfield JP, Farquhar MG (1978) Differentiation of epithelial foot processes and filtration slits: sequential appearance of occluding junctions, epithelial polyanion, and slit membranes in developing glomeruli. Lab Invest 39:90–100
Roberts AB, McCune BK, Sporn MB (1992) TGF-β: regulation of extracellular matrix. Kidney Int 41:557–559
Thiery JP, Duband JL, Dufour S, Savagner P, Imhof BA (1989) Role of fibronectin in embryogenesis. In: Mosher DF (ed) Biology of extracellular matrix: fibronectin. Academic Press, San Diego, pp 181–212
Kagami S, Border WA, Miller DE, Noble NA (1994) Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-β expression in rat glomerular mesangial cells. J Clin Invest 93:2431–2437
Bagby SP, Kirk EA, Mitchell LH, O’Reilly MM, Holden WE, Stenberg PE, Bakke AG (1993) Proliferative synergy of angiotensin II and EGF in porcine aortic vascular smooth muscle cells. Am J Physiol 265:F239–F249
Fernandez LA, Twickler J, Mead A (1985) Neovascularization produced by angiotensin II. J Lab Clin Med 105:141–145
Gomez RA (1990) Molecular biology of components of the renin-angiotensin system during development. Pediatr Nephrol 4:421–423
Gomez RA, Lynch KR, Sturgill BC, Elwood JP, Chevalier RL, Carey RM, Peach MJ (1989) Distribution of renin mRNA and its protein in the developing kidney. Am J Physiol 257: F850–F858
Millan MA, Carvallo P, Izumi S, Zemel S, Catt KJ, Aguilera G (1989) Novel sites of expression of functional angiotensin II receptors in the late gestation fetus. Science 244:1340–1342
Tufro-McReddie A, Harrison JK, Everett AD, Gomez RA (1993) Ontogeny of type 1 angiotensin II receptor gene expression in the rat. J Clin Invest 91:530–537
Grady EF, Sechi LA, Griffin CA, Schambelan M, Kalinyak JE (1991) Expression of AT2 receptors in the developing rat fetus. J Clin Invest 88:921–933
Du Y, Yao A, Guo D, Inagami T, Wang DH (1995) Differential regulation of angiotensin II receptors subtypes in rat kidney by low dietary sodium. Hypertension 25:872–877
Coimbra TM, Janssen U, Gröne HJ, Ostendorf T, Kunter U, Schmidt H, Brabant G, Floege J (2000) Early events leading to renal injury in obese Zucker (fatty) rats with type II diabetes. Kidney Int 57:167–182
Kliem V, Johnson RJ, Alpers CE, Yoshimura A, Couser WG, Koch KM, Floege J (1996) Mechanisms involved in the pathogenesis of tubulointerstitial fibrosis in 5/6-nephrectomized rats. Kidney Int 49:666–678
Stambe C, Atkins RC, Hill PA, Nikolic-Paterson DJ (2003) Activation and cellular localization of the p38 and JNK MAPK pathways in rat crescentic glomerulonephritis. Kidney Int 64:2121–2132
Hartree EF (1972) Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem 48:422–427
Fuehr Y, Kaczmarczk Y, Kruttgen GD (1955) Eine einfache colorimetrische Methode zur Inulin-Bestimmung für Nieren-Clearance-Untersuchungen bei Stoffwechselgesunden und Diabetikern. Klin Wochenschr 33:729–730
Alpers CE, Hudkins KL, Gown AM, Johnson RJ (1992) Enhanced expression of “muscle-specific” actin in glomerulonephritis. Kidney Int 41:1134–1142
El Nahas AM, Muchaneta-Kubara EC, Zhang G, Adam A, Goumenos D (1996) Phenotypic modulation of renal cells during experimental and clinical renal scarring. Kidney Int 54:S23–S27
Geleilete TJ, Melo GC, Costa RS, Volpini RA, Soares TJ, Coimbra TM (2002) Role of myofibroblasts, macrophages, transforming growth factor-beta, endothelin, angiotensin-II, and fibronectin in the progression of tubulointerstitial nephritis induced by gentamicin. J Nephrol 15:633–642
Johnson RJ, Alpers CE, Yoshimura A, Lombardi D, Pritzl P, Floege J, Schwartz SM (1992) Renal injury from angiotensin II-mediated hypertension. Hypertension 19:464–474
Chen Y, Lasaitiene D, Gabrielsson BG, Carlsson LM, Billig H, Carlsson B, Marcussen N, Sun XF, Friberg P (2004) Neonatal losartan treatment suppresses renal expression of molecules involved in cell-cell and cell-matrix interactions. J Am Soc Nephrol 15:1232–1243
Johnson RJ, Iida H, Alpers CE, Majesky MW, Schwartz SM, Pritzl P, Gordon K, Gown AM (1991) Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest 87:847–858
Carey AV, Carey RM, Gomez RA (1992) Expression of alpha-smooth muscle actin in the developing kidney vasculature. Hypertension 19 [Suppl]:168–175
Naruse K, Fujieda M, Miyasaki E, Hayashi Y, Toi M, Fukui T, Kuroda N, Hiroi M, Kurashige T, Enzan H (2000) An immunohistochemical study of developing glomeruli in human fetal kidneys. Kidney Int 57:1836–1846
Omori S, Hida M, Ishikura K, Kuramochi S, Awazu M (2000) Expression of mitogen-activated protein kinase family in rat renal development. Kidney Int 58:27–37
Hsuch WA, Do YS, Anderson PW, Law RE (1995) Angiotensin II in cell growth and matrix production. Adv Exp Med Biol 377:217–223
McCausland JE, Ryan GB, Alcorn D (1996) Angiotensin converting enzyme inhibition in the postnatal rat results in decreased cell proliferation in the renal outer medulla. Clin Exp Pharmacol Physiol 23:552–554
Guron G, Friberg P (2000) An intact renin angiotensin system is a prerequisite for normal renal development. J Hypertens 18:123–137
Nicolantonio RD, Spargo S, Morgan TO (1987) Prenatal high salt diet increases blood pressure and salt retention in spontaneously hypertensive rat. Clin Exp Pharmacol Physiol 14:233–235
Silva AA, Noronha IL, Oliveira IB de, Malheiros DMC, Heimann JC (2003) Renin-angiotensin system function and blood pressure in adult rats after perinatal salt overload. Nutr Metab Cardiovasc Dis 13:133–139
Hazon N, Parker C, Leonard R, Henderson IW (1988) Influence of an enriched sodium chloride regime during gestation and suckling and post-natally on the ontogeny of hypertension in the rat. J Hypertens 6:517–524
Acknowledgements
The authors thank Cleonice G.A. da Silva, Erika Delloiagono, and Rubens Fernando de Melo for expert technical assistance. Ana Paula C. Balbi was a recipient of Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, DF, Brazil, fellowship, and Dr. Roberto Silva Costa and Dr. Terezila Machado Coimbra are recipients of Conselho Nacional de Desenvolvimento Científico e Tecnológico, DF, Brazil, fellowships. These results were presented in abstract form at the American Society of Nephrology Meeting, Philadelphia, Pa., November 2002.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balbi, A.P.C., Costa, R.S. & Coimbra, T.M. Postnatal renal development of rats from mothers that received increased sodium intake. Pediatr Nephrol 19, 1212–1218 (2004). https://doi.org/10.1007/s00467-004-1586-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-004-1586-x