Abstract
Mortality statistics of young adults with childhood-onset end-stage renal disease (ESRD) show that cardiovascular disease (CVD) is responsible for most deaths on dialysis and after transplantation. This is most likely explained by the presence of a multitude of traditional and non-traditional risk factors in uremia, promoting the combination of classical atherosclerosis, uremic vasculopathy, and uremic cardiomyopathy. Vascular (arterial) calcifications occur with a high prevalence in young adults and their presence correlates with non-traditional risk factors, markers of inflammation, intake of calcium-containing phosphate binders, and the calcium-phosphorus product in serum. This might be explained by a high positive calcium and phosphorus balance in ESRD patients, which may be comparatively higher in the young. In addition, treatment with active vitamin D preparations may enhance the positive calcium and phosphorus balance and have a direct calcifying effect on the arterial wall. The biological process of vascular calcification resembles osteogenesis. These data indicate that vascular calcifications are related to non-traditional risk factors, inflammatory mechanisms, and disturbances in calcium and phosphorus metabolism in uremia. They provide strong evidence for a change in the current management of renal osteodystrophy in children and adolescents with ESRD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1990, Milliner et al. [1] published the first systematic study on the prevalence and distribution of soft tissue calcifications in pediatric patients with end-stage renal disease (ESRD). These authors retrospectively reviewed clinical, biochemical, and autopsy data of 120 patients, who had been cared for at the Los Angeles Children’s Hospital between 1960 and 1983. Soft tissue calcifications were found in 72 of 120 patients (60%), and most frequently involved blood vessels, lung, kidney, myocardium, coronary arteries, central nervous system, and gastric mucosa. While the occurrence of soft tissue calcifications in children had been described before in case reports, it became apparent from this study that the prevalence of this complication of ESRD in young patients had been grossly underestimated (possibly because staining for calcium deposition with the von Kossa method is not routinely performed on autopsy). This report revealed that the arterial system and the heart could be seriously damaged by the presence of calcification at a young age in patients with ESRD. Although these findings may partially reflect difficulties with treatment in the “early days” of pediatric nephrology, it is remarkable that vitamin D therapy showed the strongest association with calcifications. Moreover, the prevalence of calcifications increased during the later years of the study (coinciding with the use of more potent active vitamin D preparations and calcium-containing phosphate binders).
Recent studies using non-invasive methods for quantification of coronary artery calcification have confirmed a very high prevalence of vascular calcification in young patients [2, 3, 4]. These studies have raised concerns about the long-term prognosis of children now being treated with ESRD in view of the epidemic of cardiovascular disease (CVD) in the adult ESRD population [5]. This review outlines the clinical significance of vascular calcifications in young ESRD patients and the underlying risk factors present in this unique population.
Epidemiological studies of CVD in young adults with childhood-onset ESRD
Mortality statistics of the last decades have shown that CVD continues to be responsible for most deaths on dialysis and after transplantation. Mortality from CVD is excessively elevated in ESRD compared with the general population and the risk is highest in the youngest patients, as shown most clearly by the USRDS data [5, 6]. To address this issue, several retrospective studies analyzing survival rates have now been carried out in children, adolescents, and young adults with ESRD since childhood. The Dutch LERIC study of 381 subjects with childhood-onset ESRD demonstrated that in the 1990s the standardized mortality rate was elevated by a factor of 21 compared with the normal Dutch population and that death from CVD was most prominent on renal replacement therapy [7]. In a study of 283 German patients with childhood onset of ESRD in Heidelberg, cardio- and cerebrovascular causes accounted for 50% of the total mortality [3]. In a study by the Hannover group of 150 German children transplanted between 1970 and 1993, patient mortality from CVD was 23%, almost 10 times as high as the occurrence of malignancies [8]. Similarly, in transplant recipients in the United States, all cardiac causes combined accounted for 35% of the total mortality of patients of 0–19 years of age [9].
Thus, emerging data from pediatric centers indicate that children and young adults with ESRD die primarily of CVD and that the mortality risk from cardiac disease is higher than that from infection or malignancy. How can this be explained?
Atherosclerosis versus uremic vasculopathy
In the general population, CVD in most patients is the result of atherosclerosis. Atherosclerosis is a multifactorial disease affecting the heart and large and medium-sized arteries, with a focal distribution, and with the characteristic appearance of lipid-rich plaques in the intima of the arterial wall. Clinical manifestations include coronary heart disease, cerebrovascular disease, aortic aneurysm, and peripheral arterial occlusive disease. This disease is slowly progressive and driven by classical and non-classical risk factors over several decades [10]. It is obviously of interest whether this process is accelerated in young patients with ESRD explaining the high mortality.
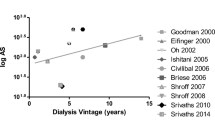
Importantly, several lines of evidence argue against an acceleration of classical atherosclerosis in ESRD. Firstly, if this process were truly accelerated, pediatric nephrologists would witness early symptoms of vascular disease, e.g., of cardiac (angina) or cerebral ischemia (stroke) on a large scale in their patients. However, young patients with ESRD usually have no clinical symptoms of ischemic heart disease, but mainly experience sudden death of cardiac origin, suggesting the presence of arrythmias or other causes [11]. Admittedly, ischemic heart disease might be underdiagnosed because it is not thought of in young patients or because of an unusual clinical presentation—the experience gained from recognizing CVD in women may serve as an example [12]. Therefore, in the absence of prospective studies, there is no firm evidence to support the clinical impression of an astonishing lack of CVD symptoms. Secondly, it is remarkable that the occurrence of vascular calcifications is not correlated with traditional risk factors for atherosclerosis, e.g., total cholesterol levels, but with non-traditional risk factors and markers of inflammation. Thirdly, the composition of coronary atherosclerotic plaques is different in adult uremic patients than in the general population, characterized by increased media thickness and marked calcification [13]. Fourthly, arterial calcifications in adult uremic subjects are not only found as part of atherosclerotic lesions involving the intima, but are also located in the media layer of the arterial wall, as in Mönckeberg calcific medial sclerosis. Moe et al. [14] have recently demonstrated the systemic presence of this form of calcification in ESRD patients by detecting medial calcifications in the inferior epigastric artery (not a usual site for atherosclerosis) in many asymptomatic patients (mean age 45 years) undergoing renal transplantation. Moreover, the occurrence of arterial calcification is a “late event” in the general population. We know from autopsy studies of hundreds of young accident victims (under 40 years) that calcium deposits emerge as granules of microscopic size in advanced lesions (type IV), which are only present in a minority of these subjects. Advanced lesions containing large amounts of calcium do not appear until the 5th decade of life [15, 16]. This is in sharp contrast to the extremely large amount of calcium in the coronary arteries of some young subjects with ESRD studied with electron beam computed tomography (EBCT) [2], again suggesting a different form of vascular disease (Fig. 1).
Since the presence of systemic calcification leads to compromised arterial elasticity, one would assume that dysfunction of the arterial wall could be measured by non-invasive means, e.g., by pulse wave velocity. Indeed pulse wave velocity is increased in uremic subjects, confirming systemic loss of arterial elasticity [7, 17]. In recent prospective studies, pulse wave velocity has emerged as a powerful predictor of mortality in adults [18, 19].
ESRD is associated with an abundance of classical and non-traditional risk factors [20], as well as with a specific form of cardiomyopathy [21, 22]. Since these risk factors most likely are additive in their effects, we believe that rather than a true acceleration of classical atherosclerosis, the combined effects of classical atherosclerosis, uremic vasculopathy, and uremic cardiomyopathy could lead to an excessively high mortality from cardiac causes at a young age (Fig. 2).
Taken together, the present data indicate that young adults have a high mortality from CVD that is characterized by the absence of typical symptoms of ischemic heart disease in most patients. The hallmarks of this vasculopathy are the high prevalence of vascular calcifications at an early age and the loss of elasticity of the arteries. This arteriopathy shows distinct morphological differences from atherosclerosis. Therefore, although prospective studies connecting vascular calcification scores with mortality are still lacking, current data suggest that vascular calcifications might be an indicator of the severity of arterial disease in young patients with ESRD.
Measurements of vascular calcifications in young patients
Following the groundbreaking study of Braun et al. [23], which demonstrated progressive coronary calcifications in adult dialysis patients, three studies have investigated coronary calcifications by EBCT and spiral CT, specifically in young patients. While the percentage of patients with calcifications was quite variable (36%, 46%, and 92%, respectively), the calcium scores in young patients with ESRD were remarkably high [2, 3, 4]. In the study by Goodman et al. [2], calcifications were associated with the calcium intake from phosphate binders, the calcium-phosphorus product, age, and the mean duration of dialysis. In the study by Oh et al. [3], which found a disturbing prevalence of calcifications of 92% in patients aged 19–39 years, predictive factors were the duration of ESRD and time on dialysis, the cumulative calcium-phosphorus product, mean intact parathyroid hormone (iPTH) plasma levels, C-reactive protein (CRP), antibodies to Chlamydia pneumoniae, and plasma homocysteine levels (Table 1).
These studies indicate a high prevalence of vascular calcifications in young adults with ESRD and a correlation with non-traditional risk factors, e.g., a high calcium-phosphorus product, high PTH levels, and markers of inflammation/infection. Importantly, vascular disease in these young patients is likely the result of ESRD, in contrast to studies in elderly adult ESRD populations where results may be confounded by age and by the burden of other individual risk factors (e.g., long-standing diabetes).
It is yet unknown to which extent this translates into cardiac morbidity and mortality in these young patients, because prospective studies in this population have not been performed. In the general population, however, a correlation of calcium measured by EBCT with atherosclerotic plaque burden has been established (in patients with coronary heart disease) [24, 25]. The amount of coronary calcium is prospectively associated with morbidity and mortality from cardiac causes [26]. Since these data were obtained in patients with atherosclerosis, i.e., vascular lesions of different composition, they may not apply to ESRD patients. Also, it is unknown whether calcifications in the intima or media can regress. Regression of atherosclerotic calcifications, however, has not been demonstrated thus far in humans. In animal experiments with monkeys fed a high-cholesterol atherogenic diet for several years (resulting in advanced intima lesions) followed by years of a low-cholesterol diet, regression of lesions was observed, with disappearance of macrophages, lipid, and foam cells, but not of calcium [16].
Risk factors for vascular calcifications: considerations in children and adolescents
Although uremic vasculopathy most likely results from the combined effect of many risk factors, those correlating with calcifications in imaging studies should be of special relevance in this population. It is obvious that exposure to a high calcium-phosphorus product, high PTH levels, or vitamin D preparations is closely related to the medical management of calcium and phosphate levels, i.e., the current approach to the treatment of renal osteodystrophy.
Calcium
Urinary calcium excretion in normal adults is about 200 mg/day. Fecal excretion varies depending on the dietary intake, but calcium balance is positive in healthy subjects <35 years. Calcium excretion diminishes with deterioration of glomerular filtration rate (GFR). Adults with ESRD, with a calculated intake of dietary calcium of 1,000 mg/day, have a positive calcium balance of about 250 mg/week [27]. If this is extrapolated to pediatric patients, who have a much higher intake of dietary calcium from food (and higher calcium requirements for bone growth), the net positive balance in ESRD might be much higher, assuming a similar calcium absorption. In the absence of firm data on the physiological requirements of calcium in uremic children, it is difficult to estimate the “true” positive calcium balance. In addition, calcium intake from phosphate binders is much higher in children. On a milligram per kilogram basis, the recommended dose for adults (calcium carbonate) is about 40–80 mg/kg, in children it is 50–200 mg/kg. In clinical studies, adults have received about 70 mg/kg per day of calcium carbonate or acetate [28]; children have received 110 mg/kg per day (range 10–340) [29]. Depending on the dialysate used, calcium balance is also positive on maintenance dialysis, ranging from 52 to 536 mg/d in adult hemodialysis patients and from 32 to 208 mg/d in peritoneal dialysis patients, respectively [27]. In addition, hypercalcemic episodes, which occur with an overall incidence of 3.5 per 100 patient-months in children with calcium carbonate treatment [29], should be considered as periods of high positive calcium balance. It is of interest that in a retrospective study concerning the safety and efficacy of calcium carbonate, these hypercalcemic episodes were associated with a reversible decline in GFR in 62% [29]. A high calcium-phosphorus product in animal models is associated with calcifications in the kidney and deterioration of GFR [30]. Finally, one has to consider the additive effect of vitamin D metabolites. It is well known that intestinal calcium resorption is regulated by active vitamin D and it can be expected that the concomitant use of vitamin D preparations dramatically increases calcium absorption in uremic patients, although studies of calcium balance in children with ESRD are lacking.
Phosphorus
Of adult dialysis patients, 70% have elevated phosphorus levels, and high serum phosphorus increases mortality risk [31]. The overall positive phosphorus balance, assuming 800–1,000 mg of dietary phosphorus/day has been calculated as 1,200/week in hemodialysis patients [27]. If extrapolated to children on a milligram per kilogram basis, this should be multiplied by a factor of 2–4, considering the current dietary recommendations for children with ESRD. It should be noted, however, that a positive phosphorus balance is needed to permit protein synthesis and growth, normal serum phosphorus levels are higher in infants and young children, and dietary recommendations in this age group include dairy products with a very high phosphate content. However, similar to the calcium intake, systematic studies of phosphorus balance in children with ESRD are lacking. The presence of hyperparathyroidism (HPT) indicates a positive balance even in incipient renal failure. Children need higher doses of phosphate binders per kilogram of body weight to prevent secondary HPT. Moreover, the concomitant use of vitamin D metabolites increases phosphorus absorption. While the age-dependent “physiological” range of the calcium-phosphorus product is unknown, and is probably higher in children than in adults, vascular calcifications in young adults correlate with the calcium-phosphorus product and the time-averaged mean intact PTH level [3].
Vascular calcifications probably occur in two types of clinical situations: (1) during periods of a high calcium-phosphorus product in serum, e.g., severe HPT with mobilization of calcium and phosphate from bone, often accompanied by an attempt to control the PTH secretion with high doses of calcitriol (enhancing at the same time calcium and phophate resorption) and (2) periods of low bone turnover, mostly induced by oversuppression of PTH secretion with active vitamin D metabolites and accompanied by hypercalcemia. It is conceivable that in these situations, the arterial wall is taking up calcium that is either mobilized from bone or cannot be metabolized by bone. In both cases, calcification, or rather ossification, is taking place in the artery instead of in the bone [14].
Vitamin D
It follows that treatment with active vitamin D preparations, although necessary to prevent rickets and assure bone growth in uremic children, is a journey between Scylla and Charybdis. Active vitamin D metabolites, e.g., calcitriol, could promote vascular calcifications not only by increasing intestinal calcium and phosphorus absorption, or by arresting calcium delivery to bone (low bone turnover), but also by direct effects on the vascular wall. Recent studies have shown that the process of mineralization is highly regulated in smooth muscle cells, the main cell type of the media. It seems that this process results from the balance of many factors either promoting or preventing mineralization, and under normal conditions is essentially inhibited [32]. Under conditions of calcification, however, vascular smooth muscle cells can undergo a phenotypic transition to osteoblast-like cells, expressing several genes involved in osteogenesis [33]. With transition, these cells express the “core binding factor alpha 1” (Cbfa1), which is an osteoblast-specific transcription factor regulating the expression of multiple genes in the osteoblast [34]. In vitro, both an increase in extracellular phosphorus [34] and uremic serum [35] are able to induce calcium deposition, increased expression of Cbfa1 and an osteoblast phenotype. This could lead to the secretion of an osteoid-like extracellular matrix that contains osteopontin and osteocalcin, i.e., bone formation in the arterial wall [34].
Vascular smooth muscle cells express the vitamin D receptor and upregulate the receptor if excessive doses of calcitriol are administered to animals [36]. In vitro, calcitriol increases expression of alkaline phosphatase, an enzyme involved in osteogenesis, and calcium deposition in these cells [37]. However, calcitriol does not seem to promote calcification by upregulation of Cbfa1 [38], but by modulating secretion of PTH-RP [37] or by other yet unknown mechanisms (e.g., upregulation of other promoters or downregulation of inhibitors). In animal experiments it was shown that media calcifications can be produced by feeding rats with vitamin D3 [39].
Growth
The process of vascular calcification resembles osteogenesis, and in view of the regulation of bone formation by growth hormone and insulin-like growth factors (IGF), respectively, the question of whether growth itself might have an effect on the occurrence of vascular calcifications is obviously important. Growth hormone and IGF-1 stimulate the proliferation of endothelial cells and smooth muscle cells in vitro and increase local IGF-1 gene expression in vascular cells [40]. Interestingly, in a rat model of arterial calcifications using high-dose vitamin D and warfarin, calcification is dependent on age and explained by growth and serum phosphate levels [41].
In human growth hormone deficiency, treatment with recombinant growth hormone (rhGH) improves the lipoprotein profile, normalizes endothelial dysfunction, reduces monocyte activation, decreases carotid intima media thickness, and increases left ventricular function [42, 43]. These are clearly beneficial effects with respect to CVD risk. However, it is yet unknown whether rhGH treatment of children with ESRD has any effect on arterial calcification.
Inflammation/infection
In the study by Oh et al. [3], the presence of coronary artery calcifications was closely correlated with CRP. In a multivariate analysis, CRP and plasma homocysteine levels remained as independent predictors (Table 1) in a model, together with iPTH and the calcium-phosphate product, explaining 75% of the variation in coronary artery calcification [3]. This indicates that inflammatory processes play a major role in the pathogenesis of these vascular changes. CRP levels are significant predictors of mortality in adult hemodialysis [44, 45] and peritoneal dialysis [46] patients.
Inflammation in this population is closely connected to oxidative stress, malnutrition, and hypoalbuminemia [47], which in turn influence plasma homocysteine levels. It is therefore yet unknown whether homocysteine levels are markers of this process or directly involved in vascular damage [48]. Interestingly, a recent study of 312 adult hemodialysis patients [49] identified a potential missing link between inflammation and calcification. Ketteler et al. [50] demonstrated a significant association of survival on dialysis with serum concentrations of fetuin-A. Fetuin is a circulating inhibitor of calcification in vivo and is downregulated during the acute-phase response [50]. It is conceivable that persistently low concentrations of this glycoprotein during chronic low-grade inflammation in uremic patients could facilitate calcification. This notion is further supported by the observation of an impaired ex vivo capacity (of sera from patients with low fetuin concentrations) to inhibit calcium-phosphate precipitation [49]. Further studies are needed to clarify the role of fetuin among the multiple pathways leading to vascular damage by inflammatory mechanisms in uremia.
Outlook
In conclusion, vascular calcifications seem to occur with a high prevalence in young adults with ESRD. It is likely that the amount of calcium in the coronary arteries in these subjects (as estimated by CT/EBCT) reflects the severity of arterial disease (risk burden of CVD) in these patients and future studies will have to prove the prognostic significance for this population. Vascular calcifications correlate with non-traditional risk factors, markers of inflammation, intake of calcium-containing phosphate binders, and the calcium-phosphorus product in serum. These factors seem to be of particular significance for young patients, possibly due to a comparatively higher positive calcium and phosphorus balance and concomitant therapy with active vitamin D preparations. The biological process of vascular calcification resembles osteogenesis [51] and may be promoted by vitamin D. Although the precise molecular mechanisms leading to calcium deposition in the arterial wall have yet to be elucidated, these data provide strong evidence for a change in the current management of renal osteodystrophy and calcium and phosphorus metabolism, respectively, in children and adolescents with ESRD. Studies evaluating new methods to decrease the calcium and phosphate burden in these patients are urgently needed (e.g., calcium-free phosphate binders, calcimimetic drugs, new vitamin D metabolites, low dialysate calcium). In addition, the prognostic significance of vascular calcifications and their potential reversibility need to be studied in this population.
References
Milliner DS, Zinsmeister AR, Lieberman E, Landing B (1990) Soft tissue calcification in pediatric patients with end-stage renal disease. Kidney Int 38:931–936
Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB (2000) Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342:1478–1483
Oh J, Wunsch R, Turzer M, Bahner M, Raggi P, Querfeld U, Mehls O, Schaefer F (2002) Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation 106:100–105
Eifinger F, Wahn F, Querfeld U, Pollok M, Gevargez A, Kriener P, Grönemeyer D (2000) Coronary artery calcifications in children and young adults treated with renal replacement therapy. Nephrol Dial Transplant 15:892–894
Foley RN, Parfrey PS, Sarnak MJ (1998) Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32:S112–S119
Parfrey PS (2000) Cardiac disease in dialysis patients: diagnosis, burden of disease, prognosis, risk factors and management. Nephrol Dial Transplant 15 [Suppl 5]:58–68
Groothoff JW, Gruppen MP, Offringa M, Hutten J, Lilien MR, Van De Kar NJ, Wolff ED, Davin JC, Heymans HS (2002) Mortality and causes of death of end-stage renal disease in children: a Dutch cohort study. Kidney Int 61:621–629
Offner G, Latta K, Hoyer PF, Baum HJ, Ehrich JH, Pichlmayr R, Brodehl J (1999) Kidney transplanted children come of age. Kidney Int 55:1509–1517
Excerpts from United States Renal Data System 1997 Annual Data Report (1997) Am J Kidney Dis 30:S1–213
Querfeld U (2001) Undertreatment of cardiac risk factors in adolescents with renal failure. Perit Dial Int 21 [Suppl 3]:S285–S291
Querfeld U (2002) Is atherosclerosis accelerated in young patients with end-stage renal disease? The contribution of paediatric nephrology. Nephrol Dial Transplant 17:719–722
Goldberg RJ, O’Donnell C, Yarzebski J, Bigelow C, Savageau J, Gore JM (1998) Sex differences in symptom presentation associated with acute myocardial infarction: a population-based perspective. Am Heart J 136:189–195
Schwarz U, Buzello M, Ritz E, Stein G, Raabe G, Wiest G, Mall G, Amann K (2000) Morphology of coronary atherosclerotic lesions in patients with end-stage renal failure. Nephrol Dial Transplant 15:218–223
Moe SM, O’Neill KD, Duan D, Ahmed S, Chen NX, Leapman SB, Fineberg N, Kopecky K (2002) Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney Int 61:638–647
Stary HC (2000) Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler Thromb Vasc Biol 20:1177–1178
Stary HC (2001) The development of calcium deposits in atherosclerotic lesions and their persistence after lipid regression. Am J Cardiol 88:16E–19E
Guerin AP, London GM, Marchais SJ, Metivier F (2000) Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant 15:1014–1021
Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM (2001) Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation 103:987–992
Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM (1999) Impact of aortic stiffness on survival in end-stage renal disease. Circulation 99:2434–2439
Chavers B, Schnaper HW (2001) Risk factors for cardiovascular disease in children on maintenance dialysis. Adv Ren Replace Ther 908:180–190
Chavers BM, Li S, Collins AJ, Herzog CA (2002) Cardiovascular disease in pediatric chronic dialysis patients. Kidney Int 62:648–653
Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE (1995) The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J Am Soc Nephrol 5:2024–2031
Braun J, Oldendorf M, Moshage W, Heidler R, Zeitler E, Luft FC (1996) Electron beam computed tomography in the evaluation of cardiac calcification in chronic dialysis patients. Am J Kidney Dis 27:394–401
Baumgart D, Schmermund A, Goerge G, Haude M, Ge J, Adamzik M, Sehnert C, Altmaier K, Groenemeyer D, Seibel R, Erbel R (1997) Comparison of electron beam computed tomography with intracoronary ultrasound and coronary angiography for detection of coronary atherosclerosis. J Am Coll Cardiol 30:57–64
Schmermund A, Baumgart D, Gorge G, Gronemeyer D, Seibel R, Bailey KR, Rumberger JA, Paar D, Erbel R (1998) Measuring the effect of risk factors on coronary atherosclerosis: coronary calcium score versus angiographic disease severity. J Am Coll Cardiol 31:1267–1273
Keelan PC, Bielak LF, Ashai K, Jamjoum LS, Denktas AE, Rumberger JA, Sheedy II PF, Peyser PA, Schwartz RS (2001) Long-term prognostic value of coronary calcification detected by electron-beam computed tomography in patients undergoing coronary angiography. Circulation 104:412–417
Hsu CH (1997) Are we mismanaging calcium and phosphate metabolism in renal failure? Am J Kidney Dis 29:641–649
Bleyer AJ, Burke SK, Dillon M, Garrett B, Kant KS, Lynch D, Rahman SN, Schoenfeld P, Teitelbaum I, Zeig S, Slatopolsky E (1999) A comparison of the calcium-free phosphate binder sevelamer hydrochloride with calcium acetate in the treatment of hyperphosphatemia in hemodialysis patients. Am J Kidney Dis 33:694–701
Clark AG, Oner A, Ward G, Turner C, Rigden SP, Haycock GB, Chantler C (1989) Safety and efficacy of calcium carbonate in children with chronic renal failure. Nephrol Dial Transplant 4:539–544
Cozzolino M, Dusso AS, Liapis H, Finch J, Lu Y, Burke SK, Slatopolsky E (2002) The effects of sevelamer hydrochloride and calcium carbonate on kidney calcification in uremic rats. J Am Soc Nephrol 13:2299–2308
Block GA, Levin NW, Port FK (1998) Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 31:607–617
Demer LL, Tintut Y (2003) Mineral exploration: search for the mechanism of vascular calcification and beyond: the 2003 Jeffrey M. Hoeg Award Lecture. Arterioscler Thromb Vasc Biol 23:1739–1743
Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM (2001) Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res 89:1147–1154
Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM (2000) Phosphate regulation of vascular smooth muscle cell calcification. Circ Res 87:E10–E17
Moe SM, Duan D, Doehle BP, O’Neill KD, Chen NX (2003) Uremia induces the osteoblast differentiation factor Cbfa1 in human blood vessels. Kidney Int 63:1003–1011
Rajasree S, Umashankar PR, Lal AV, Sarma PS, Kartha CC (2002) 1,25-Dihydroxyvitamin D3 receptor is upregulated in aortic smooth muscle cells during hypervitaminosis D. Life Sci 70:1777–1788
Jono S, Nishizawa Y, Shioi A, Morii H (1998) 1,25-Dihydroxyvitamin D3 increases in vitro vascular calcification by modulating secretion of endogenous parathyroid hormone-related peptide. Circulation 98:1302–1306
Drissi H, Pouliot A, Koolloos C, Stein JL, Lian JB, Stein GS, Wijnen AJ van (2002) 1,25-(OH)2-vitamin D3 suppresses the bone-related Runx2/Cbfa1 gene promoter. Exp Cell Res 274:323–333
Kingma JG Jr, Roy PE (1998) Ultrastructural study of hypervitaminosis D induced arterial calcification in Wistar rats. Artery 16:51–61
Bayes-Genis A, Conover CA, Schwartz RS (2000) The insulin-like growth factor axis: a review of atherosclerosis and restenosis. Circ Res 86:125–130
Price PA, Faus SA, Williamson MK (2000) Warfarin-induced artery calcification is accelerated by growth and vitamin D. Arterioscler Thromb Vasc Biol 20:317–327
Colao A, Somma C di, Cuocolo A, Spinelli L, Tedesco N, Pivonello R, Bonaduce D, Salvatore M, Lombardi G (2001) Improved cardiovascular risk factors and cardiac performance after 12 months of growth hormone (GH) replacement in young adult patients with GH deficiency. J Clin Endocrinol Metab 86:1874–1881
Pfeifer M, Verhovec R, Zizek B, Prezelj J, Poredos P, Clayton RN (1999) Growth hormone (GH) treatment reverses early atherosclerotic changes in GH-deficient adults. J Clin Endocrinol Metab 84:453–457
deFilippi C, Wasserman S, Rosanio S, Tiblier E, Sperger H, Tocchi M, Christenson R, Uretsky B, Smiley M, Gold J, Muniz H, Badalamenti J, Herzog C, Henrich W (2003) Cardiac troponin T and C-reactive protein for predicting prognosis, coronary atherosclerosis, and cardiomyopathy in patients undergoing long-term hemodialysis. JAMA 290:353–359
Wanner C, Zimmermann J, Schwedler S, Metzger T (2002) Inflammation and cardiovascular risk in dialysis patients. Kidney Int 61 [Suppl 80]:99–102
Wang AY, Woo J, Lam CW, Wang M, Sea MM, Lui SF, Li PK, Sanderson J (2003) Is a single time point C-reactive protein predictive of outcome in peritoneal dialysis patients? J Am Soc Nephrol 14:1871–1879
Pecoits-Filho R, Nordfors L, Lindholm B, Hoff CM, Schalling M, Stenvinkel P (2003) Genetic approaches in the clinical investigation of complex disorders: malnutrition, inflammation, and atherosclerosis (MIA) as a prototype. Kidney Int [Suppl] S162–S167
Suliman ME, Stenvinkel P, Barany P, Heimburger O, Anderstam B, Lindholm B (2003) Hyperhomocysteinemia and its relationship to cardiovascular disease in ESRD: influence of hypoalbuminemia, malnutrition, inflammation, and diabetes mellitus. Am J Kidney Dis 41:S89–S95
Ketteler M, Bongartz P, Westenfeld R, Wildberger JE, Mahnken AH, Bohm R, Metzger T, Wanner C, Jahnen-Dechent W, Floege J (2003) Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. Lancet 361:827–833
Schafer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, Muller-Esterl W, Schinke T, Jahnen-Dechent W (2003) The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest 112:357–366
Moe SM, Chen NX (2004) Calciphylaxis and vascular calcification: a continuum of extra-skeletal osteogenesis. Pediatr Nephrol 18:969–975
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Querfeld, U. The clinical significance of vascular calcification in young patients with end-stage renal disease. Pediatr Nephrol 19, 478–484 (2004). https://doi.org/10.1007/s00467-004-1450-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-004-1450-z