Abstract
Glomerular filtration rate (GFR) and urine and serum concentrations of cystatin C and creatinine were measured in 40 boys and 42 girls. The fractional excretion of cystatin C (FE Cyst C) increased in proportion to the decrease in GFR. Since serum creatinine concentration (S-Creatinine) in the numerator of the fractional excretion equation and serum cystatin C concentration (S-Cystatin C) in the denominator have similar numerical values, they cancel out. The result is an equation in which the FE Cyst C is equal to the ratio of urinary cystatin C to urinary creatinine (u[cystatin-C/Cr]). The ratio of u[cystatin C/Cr] was compared with GFR. Using a receiving operating characteristic (ROC) plot, the data showed that a ratio of u[cystatin C/Cr]*100 that is ≥0.100 has a sensitivity of 90.0% for identification of children with GFR ≤60 ml/min per 1.73 m2. The false-positive rate is 16.1%. The u[cystatin C/Cr] ratio is a reliable screening tool for detecting decreased GFR that does not require a serum sample.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There have been many studies in which the serum concentration of cystatin C (S-Cystatin C) and of serum creatinine (S-Creatinine) were compared as parameters of glomerular filtration rate (GFR) [1, 2, 3, 4, 5, 6, 7]. However, little attention has been focused on urinary cystatin C excretion rates, probably because of the low concentrations in urine and concern that enzymes in urine may degrade this protein [8]. In 1997 Tian et al. [9] reported that urinary cystatin C excretion was significantly increased in adults with decreased creatinine clearance (C Cr). This study showed that the fractional excretion of cystatin C (FE Cyst C) increased in proportion to the decrease in C Cr. In the present study we compared the FE Cyst C with GFR in pediatric patients. We noted that S-Cystatin C, which is in the numerator of the fractional excretion equation, and S-Creatinine, which is in the denominator, have similar numerical values. Since the ratio of these serum values is close to unity, we hypothesized that the revised equation for FE Cyst C, FE Cyst C=u[Cyst C/Cr], could serve as a parameter for GFR.
Patients and methods
Children attending the Children’s Kidney Center who were to have GFR measured for clinical purposes were recruited to provide a urine specimen for measurement of the concentration of cystatin C and of creatinine prior to starting the timed urine collection for the clearance study. The study was approved by the Pediatric Institutional Review Board of the Children’s Mercy Hospital. The purpose of the study was explained to the children and the responsible adult. The children or the adult (or both) signed the assent form prior to the study. Eighty-two children, 40 boys and 42 girls, ranging in age from 6.1 to 21.3 years (14.0±3.7 years) participated. The diagnoses among these children are shown in Table 1.
Clean-voided specimens of urine were collected from all of the children except the 3 on a catheterization program, from whom a catheterized specimen was obtained. Samples of at least 15 ml were required. The specimens were refrigerated immediately and placed in ice for transfer to the renal laboratory. The pH was adjusted to between 6.9 and 7.2 and the specimen was then heated at 56°C for 20 min. The urine pH was adjusted using 1 M sodium hydroxide or 1 M hydrochloric acid. A volume correction was used in the final calculation if the dilution factor was ≥1.01.
Table 2 shows the stability of cystatin C added to a series of fresh samples of urine to attain levels from 0.1 to 0.6 mg/l. A portion was analyzed immediately and a portion was heated in a 56°C water bath for 20 min and then analyzed. This procedure was chosen because it should provide high enough temperature and exposure time to destroy enzyme activity without affecting protein solubility. Cystatin C levels were measured by direct analysis. Five experiments were run with a total of 46 separate specimens. The results indicate that heating urine at 56°C for 20 min does not affect the reaction with the immunoprecipitation reagent (Table 2). Cystatin C in specimens so treated are stable for up to 7 days refrigerated and for at least 6 weeks frozen at −20°C.
Serum and urine cystatin C concentrations were measured using an adaptation of the DAKO Cystatin C particle-enhanced turbidimetric assay for serum and plasma. A direct assay was found to be satisfactory for urinary cystatin C concentrations to 0.03 mg/l. A concentration procedure was developed for specimens with a cystatin C concentration ≤0.03 mg/l using an Amicon Centricon-3 membrane filter with a molecular size cut-off of 10,000 daltons. The concentrating procedure yielded recovery of 102% in control samples with an assigned value of 0.031 mg/l (mean=0.0315, SD=0.0023, CV=7.4%). Cystatin C values measured on concentrated specimens were valid to a level of 0.01 mg/l. Urine specimens with cystatin C concentrations less than 0.01 mg/l were assumed to have a concentration of 0.01 mg/l for calculation of the u[cystatin C/Cr] ratio. The concentration of creatinine in the urine samples was measured using a kinetic adaptation of the Jaffé method [10]. Measurements were performed on either a Roche Cobas Classic or a Roche Mira S automated chemistry instrument. Side-by-side studies have shown close correlation of values for serum and urine creatinine and serum and urine cystatin C measured on the two instruments.
GFR was measured using the cimetidine protocol [11]. C Cr is measured in children who have received cimetidine during the preceding 2 days. During the 24 h prior to the clearance study, the children are placed on a diet free of meat, fish, and fowl, substances that contain preformed creatinine and creatinine precursors. A final dose of cimetidine, half of the daily dose, is given approximately 2 h before beginning the timed urine collection for the clearance study. The children voided or were catheterized prior to beginning the timed urine collection, and an aliquot of this specimen was used for measurement of cystatin C and creatinine concentrations. A single blood specimen was obtained during or following the timed urine collection for measurement of S-Cystatin C and S-Creatinine.
Statistical methods
The data were analyzed using SPSS 11.0 for Windows. The FE Cyst C and the u[cystatin C/Cr]*100 ratio were correlated with GFR using linear regression. The ratio of u[cystatin C/Cr] was multiplied by 100 to avoid two zeros preceding the initial integer in the calculations. The relationship between the FE Cyst C and u[cystatin C/Cr]*100 was examined using a Bland-Altman plot of the relationship between the difference and the mean after logarithmic transformation [12]. Receiver operating characteristic (ROC) plots were derived using u[cystatin C/Cr]*100 ratios with the affected individuals defined as those with a GFR of ≤70, ≤60, and ≤50 ml/min per 1.73 m2 [13]. The sensitivity and specificity with a cut-off value for a U[cystatin C/Cr]*100 ratio of 0.100 was determined for each of the three GFR levels.
Results
Table 3 shows the age, weight, height, surface area, body mass index, cimetidine GFR, and calculated GFR. The calculated GFR was derived using the equation GFR=k*L/[Cr]s. L is the height in centimeters and k values, as recommended by Schwartz, were determined in our laboratory [14]. The k values were 0.5 for all girls and boys <13 years of age and 0.6 for boys older than 13 years. Table 4 summarizes the pertinent creatinine and cystatin C data among the 82 children studied. The footnote shows that the ratio of [Cr]s/[Cyst C]s=1.04.
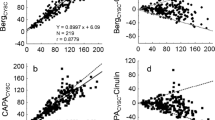
Figures 1 and 2 are the linear-log plots of FE Cyst C versus GFR and of the u[cystatin C/Cr]*100 ratio versus GFR. The plots are similar (r=0.359 and 0.402, respectively). Figure 3 is the linear regression of FE Cyst C with u[cystatin C/Cr]*100 ratios after logarithmic transformation. There is very close correlation (r=0.995). Figure 4 is the Bland-Altman plot of the difference between the FE Cyst C and the u[cystatin C/Cr]*100 ratio plotted against the average of the two after logarithmic transformation [12]. Of the 82 u[cystatin C/Cr]*100 ratios, only 2 deviated by more than 2 SD from the mean, indicating that the u[cystatin C/Cr]*100 ratio closely reflects the FE Cyst C. Figure 5 is the ROC plot of the u[cystatin C/Cr]*100 ratios with those affected defined by a GFR ≤60 ml/min per 1.73 m2. The area under the ROC plot is 0.871. The arrow points to the site on the ROC curve that is the locus of a u[cystatin C/Cr]*100 ratio of 0.100. A ratio ≥0.100 has a sensitivity of 90.0% for identification of children with GFR ≤60 ml/min per 1.73 m2. The false-positive rate is 16.1%. Table 5 shows the ROC plot data and the sensitivity and false positive-values with those affected defined by GFR ≤50, ≤60, and ≤70 ml/min per 1.73 m2 using a u[cystatin C/Cr]*100 ratio of 0.100 for the cut-off. The 95% confidence intervals for the sensitivities are shown.
Discussion
There is wide interest in the prevalence of chronic kidney disease (CKD) and of decreased kidney function because of the possibility that intervention may reduce or slow the progression to the need for dialysis or transplantation [15]. A convenient and inexpensive screening test that identifies impaired kidney function in asymptomatic individuals should be beneficial to the individual and to society. Currently, screening of an asymptomatic adult for reduced kidney function involves obtaining a personal and a family history, anthropometric data, measurement of blood pressure, urinalysis, and a blood specimen for S-Creatinine (urea nitrogen for some calculations) and cholesterol. A number of equations have been recommended for estimation of renal clearance among adults [16, 17]. Each of these equations requires a blood specimen for measurement of S-Creatinine.
A recent article based on the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative was written with the goal of improving the detection and management of CKD in children and adolescents [18]. The level of GFR was taken as the primary focus in the guideline, “because GFR provides the best measure of overall kidney function.” The recommendation was that GFR be estimated from prediction equations that take into account the patients height and gender and S-Creatinine concentration [19, 20]. GFR calculated from the prediction equations using S-Creatinine, height, and gender is a screening test for GFR not a measurement. If a screening test indicates decreased GFR, this should be checked using a reliable measurement of GFR before subjecting the child to an in-depth evaluation of kidney function. The data of the present investigation show that a specimen of urine for measurement of the ratio of cystatin C/creatinine has a 90% specificity for identification of pediatric patients with GFR ≤60 ml/min per 1.73 m2 with a false-positive rate of 16.1%. This screening test for GFR does not require a blood specimen. If this test is shown to be valid for estimation of GFR in children on a free diet who are not taking cimetidine, it will be an ideal screening test to detect asymptomatic children with reduced kidney function.
High doses of steroid given to renal transplant patients resulted in higher S-Cystatin C levels than in other children with similar GFR [21]. None of the children in this study were renal transplant recipients nor had any been on high-dose steroid therapy. Fricker et al. [22] found that thyroid hormone levels directly correlate with S-Cystatin. No child participating in this study was clinically hyper- or hypothyroid. Massive proteinuria (1,000-fold higher than that of controls) induced in Sprague-Dawley rats inhibited the tubular reabsorption of low-molecular-weight proteins [23]. Only 1 child in this study, 1 of the 9 with IgA nephropathy, had marked proteinuria. He had a urinary protein/Cr ratio of 3.18 and protein excretion calculated as 3,171 mg/24 h. He was asymptomatic with no evidence of edema or ascites.
GFR in the children in this study was measured using the cimetidine protocol [11]. The children received cimetidine for 2 days prior to and on the day of the clearance study. It has been shown that creatinine excretion rates are not altered by the ingestion of cimetidine as used in this protocol, so that should not be a factor in the employment of the u[cystatin C/Cr] ratio to detect decreased GFR [24]. The children were on a diet free of meat, fish, and fowl for 24 h prior to urine collection for the clearance study [11]. Dietary intake influences the renal rate of excretion of creatinine because the creatine pool size may be increased by diet, especially meat [25]. The ingestion of food containing preformed creatinine and creatinine precursors increases the creatinine excretion rate and causes a transient increase in S-Creatinine levels [26]. No studies have been conducted to determine if the u[cystatin C/Cr] ratio will detect decreased GFR in children on a free diet. The next step in our studies will be to evaluate the u[cystatin C/Cr] ratio in children on a free diet who are not receiving cimetidine.
In conclusion, the ratio of urinary cystatin C to urinary creatinine has been shown to be a reliable screening tool to identify children with reduced GFR when the filtration rate was measured using the cimetidine protocol [11]. If the test is found to be reliable for detecting reduced GFR in children on a free diet who are not receiving cimetidine, it should be an ideal tool for screening asymptomatic children and adolescents for CKD.
References
Filler G, Witt I, Priem F, Ehrich HH, Jung K (1997) Are cystatin C and β2-microglobulin better markers than serum creatinine for prediction of a normal glomerular filtration rate in pediatric patients? Clin Chem 43:1077–1078
Harmoinen APT, Kouri TT, Wirta OR, Lehtimäki TJ, Rantalaiho V, Turjanmaa VMH, Pasternack AI (1999) Evaluation of plasma cystatin C as a marker for glomerular filtration rate in patients with type 2 diabetes. Clin Nephrol 52:363–370
Norlund L, Fex G, Lanke J, Schenck HV, Nilsson JE, Leksell H, Grubb A (1997) Reference intervals for the glomerular filtration rate and cell-proliferation markers: serum cystatin C and serum β2-microglobulin/cystatin C-ratio. Scand J Clin Lab Invest 57:463–470
Bökenkamp A, Domanetzki M, Zinck R, Schumann G, Brodehl J (1998) Reference values for cystatin C serum concentrations in children. Pediatr Nephrol 12:125–129
Newman DJ, Thakkar H, Edwards RG, Wilkie M, White T, Grubb AO, Price CP (1995) Serum cystatin C measured by automated immunoassay: a more sensitive marker of changes in GFR than serum creatinine. Kidney Int 47:312–318
Kazama JJ, Kutsuwada K, Ataka K, Maruyama H, Gejyo F (2002) Serum cystatin C reliably detects renal dysfunction in patients with various renal diseases. Nephron 91:13–20
Kyhse-Anderson J, Schmidt C, Nordin G, Andersson B, Nilsson-Ehle P, Lindström V, Grubb A (1994) Serum cystatin C, determined by a rapid, automated particle-enhanced turbidimetric method, is a better marker than serum creatinine for glomerular filtration rate. Clin Chem 40:1921–1926
Grubb A (1992) Diagnostic value of analysis of cystatin C and protein HC in biological fluids. Clin Nephrol 38:S20–S27
Tian S, Kusano E, Ohara T, Tabel K, Itoh Y, Kawai T, Asano Y (1997) Cystatin c measurement and its practical use in patients with various renal diseases. Clin Nephrol 48:104–108
Romeo J (1975) Evaluation of a kinetic method for creatinine. Lab Med 6:15–18
Hellerstein S, Berenbom M, Alon US, Warady BA (1998) Creatinine clearance following cimetidine for estimation of glomerular filtration rate. Pediatr Nephrol 12:49–54
Altman DG, Bland JM (1983) Measurement in medicine: the analysis of method comparison studies. Statistician 32:307–317
Zweig MH, Campbell G (1993) Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 39:561–577
Schwartz GJ (1992) Does kL/Pcr estimate GFR, or does GFR determine k? Pediatr Nephrol 6:512–515
(2002) Executive summary: K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39:517–531
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 130:461–470
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Hogg RJ, Furth S, Lemley KV, Portman R, Schwartz GJ, Coresh J, Balk E, Lau J, Levin A, Kausz AT, Eknoyan G, Levey AS (2003) National Kidney Foundation’s kidney disease outcomes quality initiative clinical practice guidelines for chronic kidney disease in children and adolescents: evaluation, classification, and stratification. Pediatrics 111:1416–1421
Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A (1976) A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58:259–263
Counahan R, Chantler C, Ghazali S, Kirkwood B, Rose F, Barratt TM (1976) Estimation of glomerular filtration rate from plasma creatinine concentration in children. Arch Dis Child 51:875–878
Bökenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, Brodehl J (1999) Cystatin C serum concentrations underestimate glomerular filtration rate in renal transplant recipients. Clin Chem 45:1866–1868
Fricker M, Wiesli P, Brändle M, Schwegler B, Schmid C (2003) Impact of thyroid dysfunction on serum cystatin C. Kidney Int 63:1944–1947
Thielemans N, Lauwerys R, Bernard A (1994) Competition between albumin and low-molecular-weight proteins for renal tubular uptake in experimental nephropathies. Nephron 66:453–458
Hellerstein S, Simon SD, Berenbom M, Erwin P, Nickell E (2001) Creatinine excretion rates for renal clearance studies. Pediatr Nephrol 16:637–643
Levey AS, Perrone RD, Madias NE (1988) Serum creatinine and renal function. Annu Rev Med 39:465–490
Hellerstein S, Hunter JL, Warady BA (1988) Creatinine excretion rates for evaluation of kidney function in children. Pediatr Nephrol 2:419–424
Acknowledgements
This study was the subject of a poster presentation at the meeting of the Pediatric Academic Societies in Seattle, Washington on 4 May 2003. The study was supported in part by the Katherine B. Richardson Fund and in part by funds supplied to Stanley Hellerstein, The Ernest L. Glasscock Chair in Pediatric Research, both at The Children’s Mercy Hospital. The authors wish to express their appreciation to Carol Burns for her excellent secretarial help during accumulation of the data and in preparation of the manuscript. We also wish to acknowledge Stephen Simon, PhD, Research Biostatistician, for his contribution to the statistical evaluation of the data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hellerstein, S., Berenbom, M., Erwin, P. et al. The ratio of urinary cystatin C to urinary creatinine for detecting decreased GFR. Pediatr Nephrol 19, 521–525 (2004). https://doi.org/10.1007/s00467-003-1373-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-003-1373-0