Abstract
In patients with renal anemia, iron therapy can be administered intermittently or regularly at a low dose. We performed a randomized clinical trial in pediatric patients with end-stage renal failure on hemodialysis and absolute or functional iron deficiency. The study group received maintenance iron therapy according to the ferritin serum levels and the control group received intermittent 10-weekly doses. Success was defined as stabilization of ferritin levels between 100 and 800 μg/l and transferrin saturation (TSAT) between 20% and 50%, in addition to an increase in the hemoglobin level. The major reason for exclusion was iron overload. The study group received 6 mg/kg per month of parenteral iron [95% confidence interval (CI) 3.3–8.8] and the control group 14.4 mg/kg per month (95% CI 12–16.8) (P<0.001). After 4 months of treatment, ferritin levels increased to 66 μg/l (95% CI 69–200) in the study group and to 334 μg/l (95% CI 145–522) in the control group (P=0.009). Maintenance therapy and intermittent weekly doses were successful in 73% and 38%, respectively. After 3 months of treatment, hemoglobin levels increased to 10 g/dl, with no difference between the groups. However, in the control group the increase in hemoglobin levels was unsustained, and 3 patients needed transfusion. Patients in the control group had a higher risk of iron overload than patients in the study group (70% vs. 19%). Thus, the regimen based on assessment of serum ferritin levels was more efficient than the intermittent regimen because it increased and maintained the hemoglobin levels with lower iron doses and a lower risk of iron overload.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Treatment of renal anemia has been partially solved by the use of erythropoietin (EPO) [1, 2, 3]. Nevertheless, when there is iron deficiency, its success is reduced [4, 5, 6, 7, 8, 9, 10, 11]. Patients with chronic renal failure have high interleukin-6 levels, which modify iron metabolism [12, 13]. Due to decreased transferrin levels and the absence of EPO-driven erythropoiesis in these patients, iron is redistributed to the reticuloendothelial system and non-hemopoietic tissues. Iron is principally accumulated in hepatocytes and Kupffer cells during periods of erythropoiesis depression [14, 15, 16].
The clinical practice guidelines of the National Kidney Foundation Dialysis Quality Initiatives (NFK-DOQI) [17] have proven efficacy, but adjustments for pediatric practice may produce iron overload [18, 19]. Therefore maintenance therapy with low iron doses could be better than high intermittent doses [20, 21, 22, 23, 24, 25, 26].
Flores et al. [27] reported a 4% increase in the hematocrit and an 11% decrease in the erythropoietin requirements in children given intermittent 10-weekly doses of iron dextran regimen according to body weight. Tenbrock et al. [28] reported similar results after 3 months of treatment with 1 mg/kg per week iron dextran.
The aim of this study was to compare two regimens of iron therapy to correct iron deficiency in pediatric hemodialysis patients. The study group received a low-dose maintenance regimen according to the ferritin serum levels and the control group received intermittent 10-weekly doses.
Materials and methods
The clinical trial was carried out at the Department of Pediatric Nephrology of the Mexican Institute of Social Security from August 2000 to December 2001. The study was approved by the local ethics committee.
We included 40 pediatric hemodialysis patients with appropriate parameters for dialysis efficiency (Kt/V >1.2, urea reduction ratio >65%). They were less than 16 years, had anemia, and absolute [ferritin <100 μg/l and transferrin saturation (TSAT) <20%] or functional iron deficiency (ferritin >100 μg/l and TSAT <20% or hematocrit <33%). Patients were not eligible if they had iron overload (ferritin >800 μg/l or TSAT >50%, medium corpuscular volume >100 fl), coagulation abnormalities, abnormal hepatic function, were hypersensitive to iron, or had been transfused a month previously. Patients were excluded if they had a documented allergy to iron dextran, infection, if they were transfused with red blood cells, when they changed dialysis therapy, if they developed iron overload, or when they decided not to continue with the study. We recorded age, diagnosis, time on hemodialysis, and EPO dose.
Laboratory tests were performed monthly over 6 months. These included hemoglobin (Coulter counter), TSAT (serum iron/total iron binding capacity, measured with a spectrophotometric enzymatic assay) and ferritin (Coat-A-Count Ferritin IRMA). All intra- and inter-assay coefficients of variation were <6%.
Patients were randomized into one of two groups:
-
Study group (group 1): the iron dose depended upon the ferritin serum levels. We calculated iron reserves, iron needs, and net projected iron stores as follows: iron reserves (mg)=400x(log ferritin−log 30), iron needs for new hemoglobin synthesis: Fe (mg)=150x(11.55−Hb1), net projected iron stores=iron reserves−iron needs. The net projected iron stores were administered as weekly doses according to the body weight (<10 kg 25 mg/dose, 10–20 kg 50 mg/dose, >20 kg 100 mg/dose). After this loading dose, each patient received weekly maintenance doses of 1 mg/kg per week. Iron was discontinued if ferritin was >800 μg/l and/or TSAT >50% [19, 28, 29].
-
The control group (group 2) received ten-dose courses according to body weight: <10 kg 25 mg/dose, 10–20 kg 50 mg/dose, and >20 kg 100 mg/dose. If the hematocrit was <33%, ferritin <100 μg/l, and/or TSAT <20%, we repeated another ten-dose course [17, 27].
Iron dextran was administered intravenously in 20 ml normal saline over 30 min. Treatment was started in each patient with a test dose of 10 mg. The EPO dose was modified at 2-month intervals. If the hematocrit was ≥30%, we decreased the EPO dose by increments of 25 IU/kg per week, until 80 IU/kg per week was reached. If the hematocrit was <30%, we increased the EPO dose by 25 IU/kg per week to a maximum of 300 IU/kg per week.
The principal end-points of the study were: ferritin, TSAT, and hemoglobin levels. Success was defined as attaining and maintaining ferritin levels between 100 and 800 μg/l and attaining and maintaining TSAT levels between 20% and 50%, irrespective of the hemoglobin levels.
Statistical analysis
Results are expressed as mean±SEM. Baseline parameters of groups were compared using Student’s t and chi-squared tests. For ferritin, TSAT, and hemoglobin, a repeated-measures analysis of variance was performed. An intention-to-treat analysis was used with all the randomized patients in both groups. Success and failure were compared with the Fisher’s test. Kaplan-Meier analysis was used to calculate the hazard of iron overload in both groups. We used a continuous sequential design for monitoring trial progress and minimizing the average number of patients before stopping when the alternative hypothesis was true [30]. All data were analyzed using the NCSS 2000 and SPSS 10.0 programs.
Results
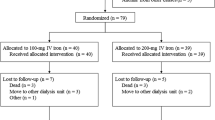
Forty children were enrolled in the study (12 female and 28 male), 20 were randomized to the study group and 20 to the control group. There were no differences in baseline parameters between groups, except for the age and time on hemodialysis. Thirteen patients had absolute iron deficiency and 27 had functional iron deficiency (Table 1). The underlying diseases were renal hypoplasia (9 patients), obstructive uropathy (8 patients), chronic glomerulonephritis (7 patients), focal segmental glomerulosclerosis (2 patients), and the etiology was unknown in 14 patients.
We excluded 6 patients in the study group and 14 in the control group. The major reason for exclusion was iron overload in 13 patients, 4 in the study group and 9 in the control group. Other exclusion causes were transfusion with red blood cells (3 patients in the control group), infection (1 in each group), and transplant (1 in each group). There were 15 patients in the study group and 16 in the control group who received treatment over at least 3 months. There were 5 and 2 patients in the study group and 6 and 1 patients in the control group who received treatment over 4 and 6 months, respectively.
The cumulative iron dose in the study group was 6 mg/kg per month [95% confidence interval (CI) 3.3–8.8] and 14.4 mg/kg per month in the control group (95% CI 12–16.8) (P<0.001, 99% power).
Ferritin increased in both groups, reaching a peak at 3 months in the study group (500–600 μg/). However, after 4 months ferritin levels were higher in the control group than in the study group (Fig. 1). TSAT was similar in the two groups after 3 months with a range of 25%–40% (P=NS, 89% power).
The basal hemoglobin was ~8 g/dl in both groups; after 3 months of treatment it increased to 10 g/dl, with no difference between the groups. However, in the control group, the increase in hemoglobin levels was intermittent, they reached normal or high levels, but decreased to subnormal levels at 6 months (Fig. 2). Hemoglobin Δ in absolute iron deficiency and in functional iron deficiency patients was 0.8 g/dl (95% CI 0.6–2.3) and 1.6 g/dl (95% CI 0.7–2.6), respectively (P=0.16, 25% power).
The average EPO dose was 197 IU/kg per week (95% CI 143–250) and 237 IU/kg per week (95% CI 200–275) in the study and control groups, respectively (P=0.18, 26% power). The mean time on hemodialysis was 16±13 months vs. 10±10 months in patients with and without iron overload (P=0.04, power 53%).
The intention-to-treat analysis with all the randomized patients in both groups revealed successful treatment in 70% of the study group and 30% of the control group (P=0.02, 73% power).
The calculated hazard of iron overload (Kaplan-Meier analysis) after 6 months of treatment was 20% in the study group and 100% in the control group (Fig. 3).
Of 13 patients with functional iron deficiency, 9 had iron overload compared with none with absolute iron deficiency (P=0.005, 87% power). The risk of iron overload was 70% in patients with functional iron deficiency in the control group compared with 19% in the study group.
Due to the ethical concern to avoid any patient in the trial receiving a treatment known to be inferior or with more side-effects (iron overload) than the other, we used a continuous sequential design for monitoring trial progress and minimizing the average number of patients before stopping when the alternative hypothesis was true. The sequential analysis with closed plan showed better results with the maintenance regimen (Fig. 4). There was no allergy to iron dextran.
Sequential analysis with closed plan. Patients enter the trial in pairs, with one assigned to each treatment. After evaluation of the response we determined according to prearranged criteria which patient in each pair responded better. When the results were plotted the upper boundary was reached after 14 paired preferences (11 favoring the study group and 3 favoring the control group). There was evidence that the maintenance regimen was better than intermittent 10-weekly doses
Discussion
The efficacy of iron treatment in patients with renal anemia has been demonstrated previously, but different iron therapies have shown different degrees of response and safety [17, 27, 28]. This reflects the variety of circumstances that modify the iron depot and mobilization in patients on dialysis.
In our study, both iron regimens increased ferritin levels, but after 4 months of treatment some patients had above-normal values. This led us to discontinue iron administration in 9 patients in the control group and 4 in the study group. After 6 months of treatment a few patients had functional iron deficiency; therefore, we continued the established dose in the study group. However, the patients in the control group could not be given iron because they had iron overload and 3 required transfusion.
Both iron therapies were equally effective in increasing hemoglobin levels to ~10 g/dl after 3 months of treatment. According to the NFK-DOQI this hemoglobin level is subnormal; however, it is sufficient to maintain the patients without the need for transfusion.
The increase in hemoglobin levels was intermittent in the control group because they reached normal or above-normal levels after 3 months of treatment, but diminished to subnormal levels after this period. This response might have deleterious effects on tissues and organs. After 6 months of treatment patients who received maintenance therapy according to the ferritin serum levels maintained their elevation in hemoglobin levels and normal iron levels. Those who received intermittent 10-weekly doses and reached iron overload had more difficulty in increasing hemoglobin levels. As Besarab et al. [22, 23] reported in adults, pediatric hemodialysis patients in the control group with high ferritin levels (>800 μg/l) did not exhibit enhanced erythropoiesis after iron overload.
Other authors regard treatment to be unsuccessful when ferritin, TSAT, or hemoglobin levels do not increase [27, 28]. In this study we decided to regard high ferritin and TSAT levels as failure because high ferritin levels have deleterious effects in many organs. Iron deposits are found in the liver, the heart, and other tissues [14]. High ferritin levels are also a marker of EPO resistance [11], morbidity, and mortality in patients with renal anemia [16]. Therefore, iron treatment must be discontinued when ferritin surpasses normal serum levels. On this basis we had to discontinue iron treatment in almost 70% of patients in the control group.
Patients with iron overload had spent longer on hemodialysis treatment. The frequent exposure to dialyzer filters stimulates the liberation of inflammatory mediators, principally interleukin-6. All these mediators alter iron metabolism. Thus, iron overload could be secondary at least in part to iron release from the reticuloendothelial system, and to decreased expression of cell surface transferrin receptors, diminishing iron uptake [12, 31]. Normally, patients with renal failure have functional iron deficiency in spite of iron overload. However, children with a long duration of hemodialysis treatment need less iron with frequent modifications of EPO doses.
We conclude that the maintenance iron regimen according to the serum ferritin levels and body weight is more effective in inducing erythropoiesis in pediatric patients with renal anemia and produces less iron overload compared with intermittent 10-weekly doses. Further studies are needed to investigate the effect of a maintenance iron regimen based on ferritin levels in peritoneal dialysis and pre-dialysis patients as well as the management of iron overload.
References
Nimer SD, Wocott DL (1991) Recombinant human erythropoietin and renal anemia: molecular biology, clinical efficacy, and nervous system effects. Ann Intern Med 114:402–416
Warady BA, Jabs K (1995) Nuevas hormonas en el arsenal terapéutico de la insuficiencia renal crónica: hormona del crecimiento y eritropoyetina. Pediatr Clin North Am 6:1466–1480
Harmon WE, Jabs KL (1999) Hemodialysis. In: Barrat TM, Avner ED, Harmon WE (eds) Pediatric nephrology, 4th edn. Lippincott Williams and Wilkins, USA, pp 1277–1279
Nissenson AR, Strobos J (1999) Iron deficiency in patients with renal failure. Kidney Int 55 [Suppl 69]:S18–S21
Van Wyck DB, Stivelman JC, Ruiz J, Kirlin LF, Katz MA, Ogden DA (1989) Iron status in patients receiving erythropoietin for dialysis-associated anemia. Kidney Int 35:712–716
Fishbane S, Frei GL, Maesaka J (1995) Reduction in recombinant human erythropoietin doses by the use of chronic intravenous iron supplementation. Am J Kidney Dis 26:41–46
Macdougall IC (1999) Strategies for iron supplementation: oral versus intravenous. Kidney Int 55 [Suppl 69]:S61–S66
Ifudu O (1998) Parenteral iron: pharmacology and clinical use. Nephron 80:249–256
Fishbane S, Mittal SK, Maesaka JK (1999) Beneficial effects of iron therapy in renal failure patients on hemodialysis. Kidney Int 55 [Suppl 69]:S67–S70
Macdougall IC, Chandler G, Orlaith E, Harchowal J (1999) Beneficial effects of adopting an aggressive intravenous iron policy in a hemodialysis unit. Am J Kidney Dis 34 [Suppl 2]:S40–S46
Seeherunvong W, Rubio L, Abitbol CL, Montane B, Strauss J, Diaz R, Zilleruelo G (2001) Identification of poor responders to erythropoietin among children undergoing hemodialysis. J Pediatr 138:710–714
Bristian BR, Khaodhiar L (1999) The systemic inflammatory response and its impact on iron nutriture in end-stage renal disease. Am J Kidney Dis 34 [Suppl 2]:S35–S39
Abbas AK, Lichtman AH, Pober JS (1999) Inmunología celular y molecular, 3er edn. Interamericana McGraw-Hill, pp 234–253, 275–308
Eschbach JW, Adamson JW (1999) Iron overload in renal failure patients: changes since the introduction of erythropoietin therapy. Kidney Int 55 [Suppl 69]:S35–S43
Park L, Uhthoff T, Tierney M, Nadler S (1998) Effect of an intravenous iron dextran regimen on iron stores, hemoglobin, and erythropoietin requirements in hemodialysis patients. Am J Kidney Dis 31:835–840
Kalantar-Zadeh K, Don BR, Rodríguez RA (2001) Serum ferritin is a marker of morbidity and mortality in hemodialysis patients. Am J Kidney Dis 37:564–572
National Kidney Foundation-Dialysis Outcomes Quality Initiative (1997) Clinical practice guidelines for the treatment of anemia of chronic renal failure. Am J Kidney Dis 30:192–240
Tarng DCH, Huang TP, Chen TW, Yang WCH (1999) Erythropoietin hyporesponsiveness: from iron deficiency to iron overload. Kidney Int 55 [Suppl 69]:S107–S118
Morgan HEG, Gautam M, Geary DF (2001) Maintenance intravenous iron therapy in pediatric hemodialysis patients. Pediatr Nephrol 16:779–783
Macdougall IC, Cavill I, Hulme B, Bain B, McGregor E, McKay P, Sanders E, Coles GA, Williams JD (1992) Detection of functional iron deficiency during erythropoietin treatment: a new approach. BMJ 304:225–226
Owen WF, Szczech L, Johnson C, Frankenfield D (1999) National perspective on iron therapy as a clinical performance measure for maintenance hemodialysis patients. Am J Kidney Dis 34 [Suppl 2]:S5–S11
Besarab A, Kaiser J, Frinak S (1999) A study of parenteral iron regimens in hemodialysis patients. Am J Kidney Dis 34:21–28
Besarab A, Amin N, Ahsan M, Vogel SE, Zazuwa G, Frinak S, Zazra JJ, Anandan JV, Gupta A (2000) Optimization of epoetin therapy with intravenous iron therapy in hemodialysis patients. J Am Soc Nephrol 11:530–538
Sepandy F, Kindal K, West M, Hirsch D (1996) Economic appraisal of maintenance parenteral iron administration in treatment of anaemia in chronic haemodialysis patients. Nephrol Dial Transplant 11:2319–2322
Taylor JE, Peat N, Porter C, Morgan AG (1996) Regular low-dose intravenous iron therapy improves response to erythropoietin in haemodialysis patients. Nephrol Dial Transplant 11:1079–1083
St Peter WL, Lambrecht LJ, Macres M (1996) Randomized cross-over study of adverse reactions and cost implications of intravenous push compared with infusion of iron dextran in hemodialysis patients. Am J Kidney Dis 28:523–528
Flores FX, Jabs K, Harmon WE, Somers MJG (1999) Safety and efficacy of intravenous iron dextran in pediatric hemodialysis patients. Pediatr Res 45:331A
Tenbrock K, Müller-Berghaus J, Michalk D, Querfeld U (1999) Intravenous iron treatment of renal anemia in children on hemodialysis. Pediatr Nephrol 13:580–582
VanWyck DB (1989) Iron management during recombinant human erythropoietin therapy. Am J Kidney Dis 14 [Suppl 1]:9–13
Pocock SJ (1993) Monitoring trial progress. In: Pocock SJ (ed) Clinical trial. A practical approach. Wiley, 142–159
Rocco MV, Bedinger MR, Milam R (2001) Duration of dialysis and its relationship to dialysis adequacy, anemia management, and serum albumin level. Am J Kidney Dis 38:813–823
Acknowledgements
We thank the staff at the hemodialysis unit at Hospital of Gineco Obstetricia 48 IMSS León, Gto. The Research was supported by CONACYT-SIHGO grant number 2000–02–02–003 and Fondo para el Fomento a la Investigación (FOFOI) del Instituto Mexicano del Seguro Social.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruiz-Jaramillo, M.C., Guízar-Mendoza, J.M., Gutiérrez-Navarro, M.J. et al. Intermittent versus maintenance iron therapy in children on hemodialysis: a randomized study. Pediatr Nephrol 19, 77–81 (2004). https://doi.org/10.1007/s00467-003-1288-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-003-1288-9