Abstract
Recently, nephrin, podocin, α-actinin, and WT1, which are located at the slit diaphragm and expressed by the podocyte, were found to be causative in congenital/familial nephrotic syndrome (NS), but their role in acquired NS remains unclear. We studied their expression in NS with the aim of disclosing their possible role in the development of proteinuria. Immunofluorescence, confocal microscopy, and image analysis were used to study the expression and the distribution in 19 children with primary NS, 9 with isolated hematuria, and 9 controls. All the children with NS presented with heavy proteinuria and foot process effacement was identified by electron microscopy. No proteinuria and foot process effacement was seen in the group with hematuria. A dramatic decrease of podocin expression was found in NS (86.66±22.74) compared with control groups (P=0.014). Furthermore, we also found the pattern of distribution of nephrin, podocin, and α-actinin changed in children with NS. In conclusion, a dramatic decrease of podocin expression and abnormal distribution of nephrin, podocin, and α-actinin were found in children with NS. No differences were found in children with isolated hematuria, suggesting involvement of these molecules in the development of proteinuria in primary NS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nephrotic syndrome (NS) characterized by heavy proteinuria, hypoalbuminemia, edema, and hyperlipidemia is one of the common renal diseases in children. Dysfunction of the glomerular filtration barrier is thought to be the main cause of proteinuria. The glomerular filtration barrier is composed of three layers, in which the slit diaphragm is the most essential unit. Recently, molecules expressed by the podocyte or located at the slit diaphragm, such as nephrin, podocin, α-actinin, and WT1, were found to be causative in congenital/familial NS [1, 2, 3, 4]. Mutations of underlying genes have been found in affected patients. Further studies have shown that these molecules themselves and their interactions are important for the integrity of the glomerular filtration barrier. The question raised is whether nephrin, podocin, α-actinin, and WT1 are involved in the development of proteinuria in non-congenital/familial NS. Thus, their expression was studied in primary NS to investigate their possible role in the pathogenesis of proteinuria.
Materials and methods
Patients
Nineteen children (12 boys and 7 girls with an average age of 8 years and 10 months) with primary NS were enrolled. No systemic diseases were found in these children based on clinical and laboratory examinations. NS was diagnosed as urinary protein >0.05 g/kg per day. All NS children (NS group) presented with heavy proteinuria clinically and the diffuse podocyte foot process effacement or fusion was identified by electron microscopy. The renal biopsies of NS children revealed minimal change nephrotic syndrome (MCNS) in 5, mesangial proliferative glomerulonephritis (MsPGN) in 10, and IgA nephropathy in 4. Mild mesangial proliferation was observed in 8 children with MsPGN and 2 children with IgA nephropathy. Mild-to-moderate mesangial proliferation was observed in 2 children with MsPGN and 2 children with IgA nephropathy. Adhesions of glomeruli with capsules in 9 of 33, 3 of 47, and 5 of 18 glomeruli were observed in 3 children with MsPGN, respectively. Cellular crescents in 3 of 33 glomeruli were observed in 1 child with MsPGN. Adhesions of glomeruli with capsules in 4 of 31 glomeruli and 4 of 20 glomeruli were also observed in 2 children with IgA nephropathy, respectively. Of 19 NS children, 18 children were treated with steroids at the time of biopsy; in 1 child steroid treatment was withdrawn 2 weeks before the biopsy. All children with MCNS, 1 of 4 children with IgA nephropathy, and 6 of 10 children with MsPGN were steroid sensitive, while others were steroid resistant. In addition to steroids, cyclophosphamide was administered to 3 children with MsPGN 1 month before renal biopsies. Of 19 NS children, only 1 child presented with an elevated serum creatinine. Some of the clinical features are listed in Table 1. In addition, 9 children with isolated hematuria clinically and non-IgA MsPGN histologically were included. No podocyte foot process effacement was observed by electron microscopy in these children (hematuria group). As controls, 9 specimens from normal portions of nephrectomized kidneys of patients with kidney tumors were used.
Immunofluorescence study
Antibodies
Primary antibodies were used as follows: polyclonal rabbit anti-human nephrin (antiserum 2965, raised against the entire extracellular part, a kind gift of Professor Karl Tryggvason, Sweden), polyclonal rabbit anti-human podocin [5] (a kind gift of Professor Corinne Antignac, France), rabbit polyclonal anti-Wilms tumor (WT) protein (C-19, Santa Cruz Biotechnology), and mouse monoclonal anti-α-actinin (clone AT6.172, Chemicon). Fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit and goat anti-mouse IgGs were used to visualize the antibody-antigen complex.
Staining procedure
The cryostat sections (5-μm) of renal biopsies were fixed with acetone at –20°C for 10 min, blocked with goat serum for 30 min, washed with phosphate-buffered saline (PBS) three times, and incubated with primary antibodies at 37°C for 3 h. After washing with PBS, the sections were incubated with secondary antibody at room temperature for 45 min, washed with PBS again, and mounted with 90% glycerol. Omission of primary antibody with PBS was used as negative control.
Confocal laser scanning microscopy
All sections were observed by a blinded reader (not knowing the clinical and histological diagnosis) under the confocal laser scanning microscope (TCS SP2, Leica, Germany). A PL APO CS 40/0.85 objective was used. The excitation wavelength of FITC was 488 nm and the emission wavelength 530 nm. Three glomeruli for each antibody and each case were taken randomly and recorded in the computer for digital image analysis (CMIAS image analysis system, Beijing, China). The average immunofluorescence intensity of three glomeruli from one patient was determined by the CMIAS system using an arbitrary scale of 256 levels of intensity to characterize the expression level of the antigen. The variation coefficient for podocin staining from 11 sections of a single control sample was 8%.
Statistical analysis
Results were expressed as mean±standard deviation (SD). The differences between groups were assessed by one-way analysis of variance. Correlation analysis was used to evaluate the significance of association between two continuous variables. All statistical analyses were performed using SPSS (10.0) analysis program.
Results
Distributions of nephrin, podocin, α-actinin, and WT1
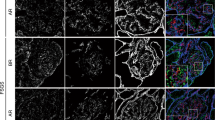
By confocal microscopy (Fig. 1), the staining of nephrin was revealed along the glomerular capillary wall as an even and linear pattern in controls. In NS, however, the distribution of nephrin was uneven throughout the glomerulus, i.e., the staining of nephrin in some parts is less than in other parts of the glomerulus and clump-like staining was seen in some areas. The distribution of nephrin in the group with hematuria was not different from controls. Podocin displayed a linear expression pattern along the glomerular capillary wall in the control and hematuria groups, but in NS showed a dotted pattern instead (Fig. 2). In NS, the expression of α-actinin differed greatly from controls, in that the staining showed a continuous pattern along the glomerular capillary wall, whereas there was a dotted line in controls. There was no difference in the expression of podocin between the hematuria and control groups. WT1 expression was restricted to the nucleus of the glomerular podocyte in controls, NS and hematuria groups. The abnormal distribution of nephrin, podocin, and α-actinin was found in 12 children with NS simultaneously. Among these, 2 children were diagnosed as MCNS, 7 children as MsPGN, and 3 children as IgA nephropathy. The renal biopsies revealed mild or mild-to-moderate mesangial proliferative changes in 10, adhesion of glomeruli with capsules in 5, and cellular crescents in 1.
Immunofluorescence staining for nephrin, podocin, α-actinin, and WT1 in different groups. The bars represent 40 μm (confocal laser scanning microscopy, original magnification ×40). A–D Nephrin, podocin, α-actinin, and WT1 expression in glomeruli of controls, respectively. E–H Nephrin, podocin, α-actinin, and WT1 expression in glomeruli of hematuria group, respectively. I–L The glomeruli showing the expression of nephrin, podocin, α-actinin, and WT1 were from a patient with IgA nephropathy
Distribution pattern of nephrin, podocin, and α-actinin in nephrotic syndrome (NS) (confocal laser scanning microscopy, original magnifications ×100). A–C Distribution pattern of nephrin, podocin, and α-actinin in the glomeruli of the control group, respectively. D–F The podocin staining in glomeruli was from an NS child with mesangioproliferative glomerulonephritis, and the nephrin and α-actinin staining in glomeruli were from a NS child with IgA nephropathy
Quantitation of results
The expression of podocin was less in NS patients than in controls (P=0.014). No significant differences were seen in the expression of nephrin, α-actinin, and WT1 among NS, hematuria, and control groups (P>0.05, Table 2). Furthermore, we compared the expression of podocin among children with different histological diagnoses. A significant decrease was seen only in NS children with MCNS and IgA nephropathy compared with the control group (P<0.01, Table 3). There was no significant difference in the expression of podocin between steroid-sensitive NS and steroid-resistant NS. An inverse correlation was found between urinary protein and the expression of podocin in NS children (r=−0.40, Fig. 3), but there was no statistical significance.
Discussion
In the past 3 years, great progress has been made in the understanding of the molecular composition of the glomerular filtration barrier. More and more molecules, such as nephrin, podocin, NEPH1 [6], P-cadherin [7], ZO-1 [8], CD2AP [9], α-actinin4, and synaptopodin [10, 11], have been identified at the slit diaphragm or at the podocyte foot process, which is bridged by the slit diaphragm. The most important molecule identified at the slit diaphragm is nephrin. Mutation of its encoding gene NPHS1 can cause lethal proteinuria at birth and lack of the slit diaphragm [12, 13, 14]. Podocin is another important molecule located at the slit diaphragm encoded by NPHS2. Mutations of NPHS2 can cause autosomal recessive steroid-resistant NS [2]. It was proposed that podocin might be important for maintaining the integrity of the slit diaphragm [15]. α-Actinins are actin-binding proteins and can attach the actin bundle to the podocyte membrane [16]. α-Actinin 4 is one of the four known isoforms and is the only isoform expressed by the kidney [3, 17, 18]. Studies have shown that α-actinin is mainly localized at the podocyte in the kidney, especially in the foot process [19, 20]. Hence, the antigen identified by anti-α-actinin antibody in kidney should be α-actinin 4. Mutations of the α-actinin 4 encoding gene, ACTN4, have been found in familial focal segmental glomerulosclerosis (FSGS). In congenital diffuse mesangial sclerosis, mutations of the WT1 encoding gene, WT1, have been found. Evidence has also shown that WT1 contributes greatly to the development of the urogenital system. A decrease of WT1 expression was also found in compromised podocytes in FSGS [21]. The above evidence highlights the importance of the slit diaphragm and the podocyte to the integrity of the glomerular filtration barrier. A defect of nephrin, podocin, α-actinin, and WT1 is the direct cause of some types of congenital/familial NS. In addition, other studies have suggested that podocin can interact with nephrin directly and strengthen the signal transduction of nephrin [15, 22]. The domain composed of nephrin and podocin may be the functional unit of the slit diaphragm. This unit may have contact with podocyte cytoskeleton proteins, such as α-actinin, through CD2AP and ZO-1 molecules [23, 24, 25, 26, 27, 28]. Studies of podocyte cell lines also suggested that the change of one podocyte molecule might affect other molecules. In this study, the expression of nephrin, podocin, α-actinin, and WT1 in the glomeruli of children with NS was studied simultaneously in order to investigate the possible mechanisms underlying proteinuria.
We found a significantly decreased expression and abnormal distribution of podocin in NS children, who presented with heavy proteinuria clinically and in whom foot process effacement in glomeruli was identified by electron microscopy. In patients with hematuria, without proteinuria and foot process effacement, no significant change of podocin was observed, suggesting the association of podocin abnormality with podocyte structural change. However, we cannot rule out the possibility that the decreased expression of podocin in some steroid-resistant NS children may be attributed to mutations of NPHS2. As an integral membrane protein, podocin can interact with the cytoskeleton and nephrin, but it also may be a molecular organizer. Hence the decrease and change in distribution can influence both the cytoskeleton and slit diaphragm. In this study, in 12 of 19 children with NS, the abnormality of podocin was accompanied by a change in distribution of nephrin and α-actinin. This was also reported in studies of cell lines. Doublier et al. [29] found that stimulation of the cytoskeleton may cause the redistribution and decrease of nephrin. Saleem et al. [28] found that disruption of the podocyte cytoskeleton can cause the redistribution of nephrin and podocin. Although we cannot conclude whether the changes of these molecules are the cause or the result of proteinuria, the abnormal distribution of nephrin, podocin, and α-actinin was related to the development of proteinuria. Our results suggest that the expression and distribution of these molecules contribute to the development of proteinuria.
In NS children, we compared the expression of podocin among different histological groups and found it to be decreased more in MCNS than in IgA nephropathy. No significant decrease of podocin was found in MsPGN. There was no significant difference in podocin expression between steroid-sensitive NS and steroid-resistant NS. No significant correlation between the urinary protein and expression level of podocin was found, but an inverse correlation was revealed. More studies are needed to evaluate the effect of the treatment on the distribution and expression of these molecules, and to investigate the relationship between the severity of proteinuria and expression of these molecules.
To date, studies on the expression of nephrin have not reached one conclusion. We studied the expression of nephrin in 73 children with heavy proteinuria using immunohistochemistry and found no significant changes in nephrin expression level [30]. Patrakka et al. [31] studied 56 patients with renal diseases by using immunofluorescence and in situ hybridization and also found no significant differences. However, Furness et al. [32] found a decrease of nephrin mRNA in three patients with MCNS. Doublier et al. [29] found a decrease and abnormal distribution of nephrin in 30 patients with proteinuria using immunofluorescence. Kim et al. [33] found that the expression pattern of nephrin in proteinuric diseases was different depending on the specific glomerular disease or the severity of glomerular damage. In this study, we found no difference in the level of nephrin expression using immunofluorescence and confocal microscopy, which was the same as our previous data. The uneven distribution of nephrin in the glomeruli of patients with NS was demonstrated. The staining of nephrin was weak in some areas but was clump-like in other areas.
In summary, a dramatic decrease and abnormal distribution of podocin expression were found in glomeruli of children with NS. Most importantly, in some NS children, the abnormal distribution of nephrin, podocin, and α-actinin was observed simultaneously, emphasizing the essential role of their interactions for the function of the glomerular filtration barrier. The mechanisms underlying this phenomenon need further study.
References
Tryggvason K (1999) Unraveling the mechanisms of glomerular ultrafiltration: nephrin, a key component of the slit diaphragm. J Am Soc Nephrol 10:2440–2445
Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C (2000) NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24:349–354
Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodríguez-Pérez JC, Allen PG, Beggs AH, Pollak MR (2000) Mutations in ACTN4, encoding α-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24:251–256
Jeanpierre C, Denamur E, Henry I, Cabanis MO, Luce S, Cecille A, Elion J, Peuchmaur M, Loirat C, Niaudet P, Gubler MC, Junien C (1998) Identification of constitutional WT1 mutations, in patients with isolated diffuse mesangial sclerosis, and analysis of genotype/phenotype correlations by use of a computerized mutation database. Am J Hum Genet 62:824–833
Roselli S, Gribouval O, Boute N, Sich M, Benessy F, Attie T, Gubler MC, Antignac C (2002) Podocin localizes in the kidney to the slit diaphragm area. Am J Pathol 160:131–139
Sellin L, Huber TB, Gerke P, Quack I, Pavenstädt H, Walz G (2003) NEPH1 defines a novel family of podocin interacting proteins. FASEB J 17:115–117
Reiser J, Kriz W, Kretzler M, Mundel P (2000) The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol 11:1–8
Schnabel E, Anderson JM, Farquhar MG (1990) The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol 111:1255–1263
Li C, Ruotsalainen V, Tryggvason K, Shaw AS, Miner JH (2000) CD2AP is expressed with nephrin in developing podocytes and is found widely in mature kidney and elsewhere. Am J Physiol Renal Physiol 279: F785–F792
Srivastava T, Garola RE, Whiting JM, Alon US (2001) Synaptopodin expression in idiopathic nephrotic syndrome of childhood. Kidney Int 59:118–125
Mundel P, Heid HW, Mundel TM, Kruger M, Reiser J, Kriz W (1997) Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol 139:193–204
Putaala H, Soininen R, Kilpelainen P, Wartiovaara J, Tryggvason K (2001) The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet 10:1–8
Ruotsalainen V, Patrakka J, Tissari P, Reponen P, Hess M, Kestilä M, Holmberg C, Salonen R, Heikinheimo M, Wartiovaara J, Tryggvason K, Jalanko H (2000) Role of nephrin in cell junction formation in human nephrogenesis. Am J Pathol 157:1905–1916
Patrakka J, Kestilä M, Wartiovaara J, Ruotsalainen V, Tissari P, Lenkkeri U, Männikkö M, Visapää I, Holmberg C, Rapola J, Tryggvason K, Jalanko H (2000) Congenital nephrotic syndrome (NPHS1): features resulting from different mutations in Finnish patients. Kidney Int 58:972–980
Schwarz K, Simons M, Reiser J, Saleem MA, Faul C, Kriz W, Shaw AS, Holzman LB, Mundel P (2001) Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest 108:1621–1629
Blanchard A, Ohanian V, Critchley D (1989) The structure and function of α-actinin. J Muscle Res Cell Motil 10:280–289
Honda K, Yamada T, Endo R, Ino Y, Gotoh M, Tsuda H, Yamada Y, Chiba H, Hirohashi S (1998) Actinin-4, a novel actin-bundling protein associated with cell motility and cancer invasion. J Cell Biol 140:1383–1393
Beggs AH, Byers TJ, Knoll JH, Boyce FM, Bruns GA, Kunkel LM (1992) Cloning and characterization of two human skeletal muscle α-actinin genes located on chromosomes 1 and 11. J Biol Chem 267:9281–9288
Lachapelle M, Bendayan M (1991) Contractile proteins in podocytes: immunocytochemical localization of actin and alpha-actinin in normal and nephrotic rat kidneys. Virchows Arch [B] 60:105–111
Drenckhahn D, Franke RP (1988) Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab Invest 59:673–682
Ohtaka A, Ootaka T, Sato H, Soma J, Sato T, Saito T, Ito S (2002) Significance of early phenotypic change of glomerular podocytes detected by Pax2 in primary focal segmental glomerulosclerosis. Am J Kidney Dis 39:475–485
Huber TB, Kottgen M, Schilling B, Walz G, Benzing T (2001) Interaction with podocin facilitates nephrin signaling. J Biol Chem 276:41543–41546
Tryggvason K, Wartiovaara J (2001) Molecular basis of glomerular permselectivity. Curr Opin Nephrol Hypertens 10:543–549
Khoshnoodi J, Tryggvason K (2001) Unraveling the molecular make-up of the glomerular podocyte slit diaphragm. Exp Nephrol 9:355–359
Kerjaschki D (2001) Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest 108:1583–1587
Yuan H, Takeuchi E, Salant DJ (2002) Podocyte slit-diaphragm protein nephrin is linked to the actin cytoskeleton. Am J Physiol Renal Physiol 282:F585–F591
Jalanko H, Patrakka J, Tryggvason K, Holmberg C (2001) Genetic kidney diseases disclose the pathogenesis of proteinuria. Ann Med 33:526–533
Saleem MA, Ni L, Witherden I, Tryggvason K, Ruotsalainen V, Mundel P, Mathieson PW (2002) Co-localization of nephrin, podocin, and the actin cytoskeleton: evidence for a role in podocyte foot process formation. Am J Pathol 161:1459–1466
Doublier S, Ruotsalainen V, Salvidio G, Lupia E, Biancone L, Conaldi PG, Reponen P, Tryggvason K, Camussi G (2001) Nephrin redistribution on podocytes is a potential mechanism for proteinuria in patients with primary acquired nephrotic syndrome. Am J Pathol 158:1723–1731
Guan N, Ding J, Zhang JJ, Xiao HJ, Liu JC, Yang JY (2001) Nephrin expression in kidneys of children with acquired renal diseases. Chin J Pediatr 39:718–721
Patrakka J, Ruotsalainen V, Ketola I, Holmberg C, Heikinheimo M, Tryggvason K, Jalanko H (2001) Expression of nephrin in pediatric kidney diseases. J Am Soc Nephrol 12:289–296
Furness PN, Hall LL, Shaw JA, Pringle JH (1999) Glomerular expression of nephrin is decreased in acquired human nephrotic syndrome. Nephrol Dial Transplant 14:1234–1237
Kim BK, Hong HK, Kim JH, Lee HS (2002) Differential expression of nephrin in acquired human proteinuric diseases. Am J Kidney Dis 40:964–973
Acknowledgements.
This study was supported by National Nature Science Foundation of China (30170992). We are grateful to Professor Karl Tryggvason (Sweden) and Corinne Antignac (France) for their kind gift of antibodies. We also thank Jianping Huang, Yong Yao, Huijie Xiao, and Jingcheng Liu for their support of this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guan, N., Ding, J., Zhang, J. et al. Expression of nephrin, podocin, α-actinin, and WT1 in children with nephrotic syndrome. Pediatr Nephrol 18, 1122–1127 (2003). https://doi.org/10.1007/s00467-003-1240-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-003-1240-z