Abstract
Introduction
Single-port laparoscopic extended right hemicolectomy with complete mesocolic excision and central vascular ligation is technically challenging, and a standardized procedure is needed to minimize technical hazards.
Technique
As a first step, the hepatic flexure is mobilized from the duodenum, and the third part of the duodenum and pancreatic head was exposed. Next, the ileocecal vessels are divided at the root using a medial-to-lateral approach, and the cecum is separated from the retroperitoneal space. This process completes the mobilization of the right colon. In the second step, the omental bursa is opened, and the inferior border of the pancreas is exposed. The mobilized right colon is turned around to the left of the superior mesenteric vein, continuing to separate the mesentery from right to left side, and the right colic vessels are divided at the roots. The inverted right colon is restored to its original position, and the mesenteric fat is dissected along the left edge of the superior mesenteric artery to the inferior border of the pancreas.
Results
A total of 57 consecutive patients with advanced hepatic flexure colon cancer (n = 24) and transverse colon cancer (n = 33) underwent S-ERHC. The conversion rate to open surgery was 5.3%. Operative time, blood loss, and number of harvested lymph nodes were 232 min (interquartile range [IQR], 184–277 min), 5 mL (IQR, 5–66 mL), and 30 (IQR, 22–38), respectively. According to the Clavien–Dindo classification, the grade ≥ 2 complication rate was 10.5%. Median duration of hospitalization was 9 days (IQR, 7–13 days).
Conclusions
Single-port laparoscopic extended right hemicolectomy using a right colon rotation technique is safe, feasible, and useful. This technique of repeating the inversion and restoration of the right colon may help avoid bleeding and damage to other organs and facilitate reliable lymph node dissection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The concept of complete mesocolic excision (CME) with central vascular ligation (CVL) has been introduced for colon cancer, and is known to result in superior oncologic outcomes [1, 2]. In advanced cancer of the hepatic flexure colon or proximal transverse colon, dissection of lymphoadipose tissue along the superior mesenteric artery (SMA) and inferior border of the pancreas has been advocated, under a concept known as D3 lymphadenectomy [3], and extended right hemicolectomy is required.
A few studies have reported the safety and feasibility of single-incision laparoscopic right hemicolectomy comparing to multi-port laparoscopic surgery [4, 5]. However, no studies have yet evaluated the clinical outcomes of single-port laparoscopic extended right hemicolectomy (S-ERHC). S-ERHC is technically challenging because of the complicated vasculature, and techniques have not been standardized. Here, we propose a novel technique that appears useful for S-ERHC.
Patients and methods
Patients
Between April 2015 and April 2019, extended right hemicolectomy for hepatic flexure colon cancer or proximal transverse colon cancer was performed on 70 patients at two hospitals, including 5 patients with open surgery, 4 with multi-port laparoscopic surgery, and 61 with single-port surgery. Among the 61 patients who underwent single-port surgery, we excluded two patients who had previously undergone ileocecal resection (n = 1) or transverse colectomy (n = 1). Two other patients with single-port surgery were excluded from analysis because they underwent simultaneous gastrectomy. A final total of 57 patients was thus evaluated in this study.
Extended right hemicolectomy with D3 lymphadenectomy
S-ERHC was performed for hepatic flexure or proximal transverse colon cancer by 3 surgeons, two of whom had experience performing more than 200 single-port surgeries. The third surgeon was a novice. We performed D3 lymph node dissection in all patients. D3 lymphadenectomy involves complete dissection of regional lymph nodes, including the pericolic lymph nodes (201, 211, and 221), intermediate lymph nodes (202, 212, 222-rt, and 222-lt), and main lymph nodes (203, 213, and 223) along the superior mesenteric arteries as defined by the Japanese Society for Cancer of the Colon and Rectum [3]. We performed lymph node dissection within the range of the D3 area, as follows: cranial margin, inferior border of the pancreas; left-side margin, left edge of the SMA; and caudal margin, root of the ileocolic artery (ICA) and vein (ICV), by ligating the root of arteries and veins belonging to the right colon (Fig. 1).
Surgical procedures
Port setting
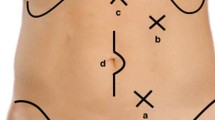
A single intra-umbilical incision of 25–30 mm was made, and an E-Z Access port device (Hakko, Nagano, Japan) was placed on the Lap Protector™ (Hakko) for insertion of two 5-mm trocars and one 12-mm trocar into an equilateral triangle, as described previously [6].
First step: mobilization of the right colon
The operating surgeon stands on the left side of the patient to start the surgery (Fig. 2A). First, we start with right colon mobilization. With the patient in a reverse Trendelenburg with right side elevated, the hepatic flexure is mobilized from the second part of the duodenum (Fig. 2B); then continuing down, the dorsal aspect of the ascending mesentery and the mesenteric root are separated from the third part of the duodenum and pancreatic head (Fig. 2C). Next, changing the surgical position to the Trendelenburg position with right side elevated, we incise the peritoneum in the mesentery at the inferior edge of the ileocolic vascular pedicles (at the lower border of the D3 area). After division of the ICA and ICV, the mesentery is mobilized sharply, preserving the right prerenal fascia and prepancreatic fascia through the mesocolic plane, continuing up to the left side of the SMA and toward the ligament of Treitz while simultaneously removing fat from the caudal side to the cranial side until reaching the gastrocolic trunk of Henle (GCT) and middle colic artery (MCA) roots. The cecum is separated from the retroperitoneal tissues. As of this point in the process, mobilization of the right colon has been completed.
Second step: D3 lymphadenectomy with CME and CVL
The operating surgeon moves between the legs of the patient, with the camera holder on the left (Fig. 3A). Changing the surgical position to the reverse Trendelenburg position with right side elevated again, the right colon is rotated from right to left around the superior mesenteric vein (SMV), continuing to carefully separate the right colic mesentery from the duodenum and pancreatic head (Fig. 3B). If the right colic artery (RCA) and right colic vein are observed at this time, they are divided at the respective roots. The greater omentum is separated from the transverse colon, and the omental bursa is opened; then the inferior border of the pancreas is exposed. Deploying the right gastroepiploic vein to the ventral side, the gastroepiploic vessels are separated from the transverse mesocolon and pancreas according to the embryological planes. If the accessory right colic vein (ARCV) is observed at this time, it is divided at the root from the anterior superior pancreaticoduodenal vein. Furthermore, separating the mesentery from right side to left side, the ARCV is divided at the root from the GCT (Fig. 3C), the middle colic vein (MCV) is divided at the root from the SMV (Fig. 3D), and the MCA is divided at the root from the SMA (Fig. 3E). The inverted right colon is restored to its original position, and the mesenteric fat is dissected from caudal to cranial along the left edge of the SMA to the inferior border of the pancreas (Fig. 3F). Repeating the inversion and restoration of the right colon according to variations in vascular anatomy is very important to avoid bleeding and damage to other organs and to achieve reliable lymph node dissection. Finally, the terminal ileum and central transverse colon are divided. An extracorporeal functional end-to-end anastomosis is then created. Drains are not used. All skin incisions are closed with absorbable sutures.
A Schematic of the positions of the operating surgeon and laparoscopist. B Rotation of the right colon from right to left around the SMV. Division of the ARCV (C), MCV (D), MCA (E) using a right colon rotation technique. F Dissection of lymphoadipose tissue from caudal to cranial along the left edge of the SMA. SMV superior mesenteric vein; ARCV accessory right colic vein; MCV middle colic vein; MCA middle colic artery; SMA superior mesenteric artery
Results
A total of 57 consecutive patients with stage I–III hepatic flexure colon cancer (n = 24) and transverse colon cancer (n = 33) who underwent S-ERHC were enrolled in this study. Patient profiles are summarized in Table 1. Median tumor size was 40 mm (IQR, 30–55 mm). The overall cohort comprised 8 patients with clinical stage I, 16 with stage II, and 33 with stage III. No patients in this study received neoadjuvant chemotherapy. Table 2 summarizes the operative findings. Three of the patients required conversion to open surgery. The reason for conversion was strong inflammatory adhesion to the small intestine in 1 patient, stomach invasion in 1 patient, and duodenal invasion in 1 patient. No conversion to multi-port laparoscopic surgery was performed in any patient. Median operative time was 232 min (IQR, 184–277 min). Median blood loss was 5 mL (IQR, 5–66 mL). The median number of harvested lymph nodes was 30 (IQR, 22–38). Median length of the skin incision was 37 mm (IQR, 30–55 mm). Table 3 summarizes the postoperative complications. According to the Clavien–Dindo classification, the grade ≥ 2 complication rate was 10.5%. Median length of hospital stay was 9 days (IQR, 7–13 days).
Discussion
In extended right hemicolectomy, the difficulty of surgery is due to the complexity of anatomy related to the mesenteric planes and vascular anatomy. In particular, the complexity of venous anatomy [7] has been increasing the risk of intraoperative bleeding, which can reduce the quality of curability. It is very important that the oncological advantages of CME not be jeopardized and the advantages of the laparoscopic approach be maintained, regardless of open, laparoscopic, or single-port laparoscopic surgery. We thus advocate a right colon rotation technique (the flip-flap method) in S-ERHC, as useful in terms of dividing colic vessels while checking the outline of lymphoadipose tissue to be dissected.
In single-port laparoscopic right hemicolectomy, medial-to-lateral [5, 8] and inferior-to-superior approaches [9] have been reported, with CVL of the middle colic and ileocolic vessels performed before separating the mesocolon from the retroperitoneal layer. A cranial approach has recently been reported for multi-port laparoscopic right hemicolectomy [10], with CVL of the middle colic vessels performed after reversal of the torsion and fusion of the transverse mesocolon. Regardless of the medial-to-lateral, inferior-to-superior, or cranial approach, the operative view is only from the cranial or caudal side, so the procedure is limited to surgical operations in front of the always-partitioned mesocolon, which may result in injury to tissues behind the partitioned mesocolon. On the other hand, open surgery has conventionally employed a lateral approach [11]. Using a lateral approach, the transverse mesocolon can be completely separated from the adjacent organs without injuring the mesocolon, before dividing the middle colic vessels. From the concept of CME, we consider that a lateral approach seems to represent the most reasonable option.
In laparoscopic surgery, a lateral approach is considered very difficult due to the limits on operative space and maneuverability of the forceps [10]. However, the right colon rotation technique can overcome this weakness. First, in this technique, the mobilized right colon can be deployed in various directions, and the optimal operative field can be developed regardless of variations in vascular anatomy. These advantages are associated with reductions in other organ injury and unexpected intraoperative bleeding. Second, the rotated right colon is physically stable and does not require dynamic development, allowing the surgeon to lightly add tension to achieve safe vascular treatment without damaging the mesentery, like a lateral approach in open surgery. Moreover, the platform attached to the umbilicus can be rotated in single-port laparoscopic surgery, and the surgery can be continued while maintaining a coaxial setting with respect to the operative targets.
Various limitations to our study need to be considered when interpreting these results. First, the study cohort was small and the investigation was retrospective in design. Second, the BMI in our cohort was typical of a Japanese population. The generalizability of our results to the higher-BMI populations of Europe or the United States is thus questionable, and BMI may significantly affect the feasibility of rotating the right colon. However, we consider that ERHC with CME and CVL for advanced hepatic flexure colon or transverse colon cancer can be performed safely and more feasibly using a right colon rotation technique.
Conclusions
Single-port extended right hemicolectomy with CME and CVL using a right colon rotation technique is safe, feasible, and useful for advanced right colon cancer.
References
Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S (2009) Standardized surgery for colonic cancer: complete mesocolic excision and central ligation–technical notes and outcome. Colorectal Dis 11:354–364
West NP, Hohenberger W, Weber K, Perrakis A, Finan PJ, Quirke P (2010) Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol 28:272–278
Japanese Society for Cancer of the Colon and Rectum (2019) Japanese classification of colorectal, appendiceal, and anal carcinoma, third. English. Kanehara & CO., Ltd., Tokyo
Song Z, Li Y, Liu K, Jiang Y, Shi Y, Ji X, Zhang T, Wu H, Shi Y, Zhao R (2019) Clinical and oncologic outcomes of single-incision laparoscopic surgery for right colon cancer: a propensity score matching analysis. Surg Endosc 33:1117–1123
Chew MH, Chang MH, Tan WS, Wong MT, Tang CL (2013) Conventional laparoscopic versus single-incision laparoscopic right hemicolectomy: a case cohort comparison of short-term outcomes in 144 consecutive cases. Surg Endosc 27:471–477
Tei M, Wakasugi M, Akamatsu H (2015) Comparison of perioperative and short-term oncological outcomes after single- or multiport surgery for colorectal cancer. Colorectal Dis 17:O141–O147
Ogino T, Takemasa I, Horitsugi G, Furuyashiki M, Ohta K, Uemura M et al (2014) Preoperative evaluation of venous anatomy in laparoscopic complete mesocolic excision for right colon cancer. Ann Surg Oncol 21(Suppl 3):S429–S435
Waters JA, Rapp BM, Guzman MJ, Jester AJ, Selzer DJ, Robb BW et al (2012) Single-port laparoscopic right hemicolectomy: the first 100 resections. Dis Colon Rectum 55:134–139
Haas EM, Pedraza R, Nieto J, Malave V (2014) Single-incision laparoscopic right hemicolectomy: inferior-to-superior approach with intracorporeal anastomosis. Surg Laparosc Endosc Percutan Tech 24:e226–e227
Matsuda T, Iwasaki T, Sumi Y, Yamashita K, Hasegawa H, Yamamoto M et al (2017) Laparoscopic complete mesocolic excision for right-sided colon cancer using a cranial approach: anatomical and embryological consideration. Int J Colorectal Dis 32:139–141
Acar HI, Comert A, Avsar A, Celik S, Kuzu MA (2014) Dynamic article: surgical anatomical planes for complete mesocolic excision and applied vascular anatomy of the right colon. Dis Colon Rectum 57:1169–1175
Acknowledgements
The authors thank Natsuki Fujita for contributing to the narration of the video.
Funding
This study was not supported by any foundations or external funding.
Author information
Authors and Affiliations
Contributions
MT and YS conceived and designed the study. MT, YS, MO, YY, TS, MI, and JH acquired data; MT and YS analyzed and interpreted the data; MT and YS drafted the manuscript; MO, YY, TS, MI, JH, and HA critically revised the article; and MO, YY, TS, MI, JH, and HA approved the final version of the manuscript to be published.
Corresponding author
Ethics declarations
Disclosure
Mitsuyoshi Tei, Yozo Suzuki, Masahisa Ohtsuka, Yukihiro Yoshiwaka, Toshinori Sueda, Mitsunobu Imazato, Junichi Hasegawa, and Hiroki Akamatsu have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 155007 KB)
Rights and permissions
About this article
Cite this article
Tei, M., Suzuki, Y., Ohtsuka, M. et al. Single-port laparoscopic extended right hemicolectomy with complete mesocolic excision and central vascular ligation using a right colon rotation technique (flip-flap method). Surg Endosc 35, 5359–5364 (2021). https://doi.org/10.1007/s00464-021-08500-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-021-08500-3