Abstract
Background
The recent development of 3D vision in laparoscopic and robotic surgical systems raises the question of whether these two procedures are equivalent. The aim of this study was to evaluate the surgical and long-term oncological outcomes of 3D laparoscopic (3D-LLR) and robotic liver resection (RLR) for hepatocellular carcinoma (HCC).
Methods
The data for operative time, morbidity, margins, and survival were reviewed for 3D-LLR and compared with RLR.
Results
From 2011 to 2017, 93 patients with HCC, including 58 (62%) with cirrhosis, underwent 3D-LLR [49 (53%)] or RLR [44 (47%)]. No difference was observed in operative time (269 vs. 252 min; p = 0.52), overall (27% vs. RLR: 16%; p = 0.49) and severe morbidity (4% vs. 2%; p = 0.77) or in the surgical margin width (9 vs. 11 mm; p = 0.30) between the 3D-LLR and RLR groups. The 3-year overall and recurrence-free survival rates after 3D-LLR and RLR were 82% and 24% and 91% (p = 0.16) and 48% (p = 0.18), respectively.
Conclusions
The 3D-LLR and RLR systems provide comparable surgical margins with similar short- and long-term oncological outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Curative surgery is the mainstay for the management of selected patients with hepatocellular carcinoma (HCC). Laparoscopic liver resection (LLR), whenever feasible, has been shown to decrease blood loss, postoperative morbidity, and hospital stay compared to an open approach without hampering long-term oncological outcome. These advantages have been acknowledged in the recent update of the EASL guidelines (European Association for the Study of the Liver) for the management of HCC [1].
In addition to intraoperative ultrasonography, optimal vision is paramount to achieve the double objective of resection: to be both curative, i.e., with a safe margin, and parenchyma-sparing to prevent postoperative liver function due to a too small remnant liver. Whereas studies comparing laparoscopic with 2D vision to robotic-assisted laparoscopic LR (see Table 1) [2] are available, no comparison of 3D laparoscopic (3D-LLR) and robotic-assisted LR (RLR) has been reported so far. The aim of the study was to compare short-term and long-term oncological outcomes following 3D-LLR or RLR in a consecutive series of patients with HCC.
Materials and methods
Study design
The present study included all consecutive patients who underwent minimally invasive liver resection for HCC at 3 Western hepatobiliary centers. All patients with HCC who underwent RLR at two hepatobiliary centers (Center 1: Henri Mondor Hospital, Créteil, France, and Center 2: University of Pisa Hospital, Pisa, Italy) from 2011 to 2017 were compared with all patients who underwent 3D-LLR at Pitié-Salpêtrière Hospital, Paris, France (Center 3) from 2015 to 2017 (the first case of 3D-LLR for HCC was performed in 2015). All procedures, regardless of the approach, were purely laparoscopic with or without robot assistance.
On an intention-to-treat basis, all patients who required conversion to the open approach were included in the analysis. This study was approved by the institutional review board in each center. All patients in this study provided informed consent.
Preoperative assessment
The diagnosis of HCC relied on previous guidelines [1]. Preoperatively, cirrhosis was diagnosed based on noninvasive criteria or histology whenever available. The confirmation of cirrhosis was obtained on the specimen analysis. All candidates with a diagnosis of HCC were discussed at multidisciplinary liver cancer meetings at each center using the same algorithms to proceed for surgery and whether to use a laparoscopic approach [2,3,4]. AFP level and portal hypertension were not considered in the decision to proceed with surgery.
Contraindications for the laparoscopic approach include large tumors or tumors close to the hepatic hilum, the inferior vena cava, or the hepatocaval confluence.
3D laparoscopic liver resection
The 3D-LLRs were performed since November 2014 [5]. The surgical technique used for 3D-LLR has previously been described in detail [5]. In brief, 3D-LLR was performed using five or six trocars and a 3D flexible laparoscope (Olympus 3D Vision System, Olympus, Tokyo, Japan) [6]. The 3D images were displayed on LCD HD screens and viewed with passive polarizing glasses (Fig. 1A). The liver parenchyma was divided using an ultrasonic dissector associated with bipolar cautery (CUSA Excel; Integra LifeSciences, USA) and a Thunderbeat scalpel (Olympus). An abdominal drainage was placed at the discretion of the surgeon.
The 3D vision system for 3D laparoscopic (A) and robotic (B) approaches. Note that for the 3D laparoscopic procedures, the assistants used 3D polarized glasses for surgery (A). For the robotic procedures, unlike the primary surgeon, who uses the 3D visual console of the robot, the surgical assistants still have to depend on 2D images projected onto a flat-screen monitor (B)
Robotic liver resection
The surgical techniques used for the robotic approach at these two centers have previously been reported [5, 7,8,9,10,11,12,13]. RLR was performed using a da Vinci robot (first version or Si da Vinci Surgical System; Intuitive Surgical Inc., Sunnyvale, CA, USA) with three or four arms: a 12-mm trocar was used for the 30-degree camera, and two or three 8-mm trocars were used for the robotic instrument arms. The robot includes a console where the operator sits while operating. The 3D images were displayed on a screen located at the surgeon console (Fig. 1B). The liver parenchyma was divided using either Harmonic curved shears (Ethicon, USA) or a combination of bipolar and monopolar energy devices [14]. All robotic procedures were purely robotic. An abdominal drainage was placed at the discretion of the surgeon.
In both approaches, an optimized CO2 insufflator with pneumoperitoneum maintenance (AirSeal™; SurgiQuest Inc., Milford, USA) was used. Similarly, the liver pedicle was tapped and intermittent vascular clamping used at the discretion of the surgeon.
Perioperative management and follow-up after surgery
Resection was defined as per the Couinaud classification [15]. In nonanatomic resection, surgery was aimed at resecting all detectable lesions with tumor-free margins ≥ 10 mm [16]. When a tumor-free margin ≥ 10 mm could not be obtained due to proximity/contact with major vasculobiliary structures, resection was still performed, provided that this surgery was macroscopically complete.
At the laparoscopic center, the Pringle maneuver was liberally applied, whereas at the robotic centers, this maneuver was applied only in cases of significant bleeding.
The following points were common to all three centers: (1) during the liver parenchyma transection, low central venous pressure was maintained (< 5 mmHg) with limited intravenous fluid administration; (2) large fluid administration, whenever needed, was performed once transection was achieved; (3) following hospital discharge, the patients were followed up at the outpatient visit at 1 month after the surgery, every 3 months for the first 2 years, and every 6 months thereafter; and (4) HCC recurrence was defined as the presence of typical radiological signs of new intra- and/or extrahepatic nodules.
Definition and outcomes
The operative time was defined as the time from incision to wound closure. The time required to dock and undock the robotic arm for RLR was included in the operative time.
Postoperative mortality was defined as mortality occurring within 90 days of surgery or at any time during the hospital stay. All complications were assessed for up to 90 days after surgery. Postoperative liver failure, ascites, biliary fistula, and hemorrhage were defined according to the International Study Group of Liver Surgery [17,18,19]. Infectious complications were diagnosed on a spectrum of clinical and/or radiological signs, along with increased levels of inflammatory markers, the positivity/negativity of fluid/blood cultures and the requirement of antibiotics or further intervention. Morbidity was graded according to the Clavien–Dindo classification system, and the most severe grade for each patient was retained [20]. Severe morbidity was defined as any complication graded III or IV. The cumulative morbidity was also measured using the comprehensive complication index (CCI ®) [21]. The CCI ® integrates all postoperative complications, including their severity, on a continuous numeric scale from 0 (no complications) to 100 (postoperative mortality).
Readmission within 90 days after hospital discharge was recorded. Overall survival (OS) was measured from resection to the last follow-up visit or death for any reason. Recurrence-free survival (RFS) was defined as the time from resection to the date of the first clinical or radiological diagnosis of HCC recurrence or death of any cause.
Statistical analysis
Categorical variables are given as numbers and percentages. Continuous variables are expressed as medians and ranges. Statistical analyses were performed using the Mann–Whitney U test for nonparametric ordinal variables and the Fisher exact test for categorical variables. Survival analysis was calculated using the Kaplan–Meier method and compared using the log-rank test. A p value < 0.05 was considered statistically significant. Statistical analyses were performed using StatView version 5.0 (SAS Institute, Inc., Cary, NC, USA). The present study complies with the Reporting of Studies Conducted Using Observational Routinely collected Health Data (RECORD) guidelines [22].
Results
The study population included 93 consecutive patients: 49 (53%) in the 3D-LLR group and 44 (47%) in the RLR group. No surgeon reported any side effects, ocular symptoms, or vision-related complaints during the study period. As shown in Table 1, relevant patient and tumor-related variables were not different between the two groups. Patients in the 3D-LLR group more often underwent a Pringle maneuver (p < 0.0001) and required less blood transfusion (p = 0.03) than did those in the RLR group. Although not significant, the conversion to an open approach was more frequently observed in the 3D-LLR group than in the RLR group (p = 0.11).
No significant difference was observed in operative time between the two groups (Table 2). When stratified according to the extent of resection, 3D laparoscopic major hepatectomy was shorter than robotic major hepatectomy (394 ± 73 vs. 509 ± 105 min; p = 0.05; Fig. 2A). When stratified according to the location of the minor resection, no difference was observed between the two procedures (p > 0.05; Fig. 2C).
The postoperative mortality was nil in both groups. The two groups were not different in terms of overall and severe morbidity rates (p = 0.77) or hospital stay (p = 0.27, Table 3). The overall median post-resection morbidity index (CCI ®) did not differ between the two groups (p = 0.22). The readmission rate was nil in both groups.
In the specimen analysis, margin width (p = 0.30) and rate of resection margin ≥ 10 mm (p = 0.40) were similar between both groups. When stratified according to the extent of resection, the surgical margin width was comparable between 3D-LLR and RLR (p > 0.05 for major and minor hepatectomies; Fig. 2B). When stratified according to the location of the minor resection, no difference was observed between the two procedures (p > 0.05; Fig. 2D).
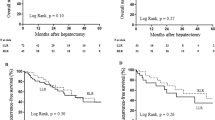
The mean follow-up period was 29 ± 19 months (3D-LLR: 26 ± 13 vs. RLR: 32 ± 24 months; p = 0.12). The 3-year OS rate was 82% in the 3D-LLR group and 91% in the RLR group (p = 0.16; Fig. 3A). The 3-year RFS rate was 24% in the 3D-LLR group and 48% in the RLR group (p = 0.18; Fig. 3B).
Discussion
The present study was the first to show that for similar consecutive patients, LLR with 3D visualization and RLR achieved similar operative times and short-term outcomes and similar safe margins and long-term oncological outcomes.
This series included two homogenous populations of patients who underwent minimally invasive LR for HCC during a recent period by three teams with expertise in laparoscopic and robotic liver resections. From 2011 to 2017, a total of 133 consecutive patients underwent RLR at the 2 robotic centers (Centers 1 and 2). Of these, 44 patients had RLR for HCC. Since the first 3D laparoscopic case performed in November 2014, a total of 187 consecutive patients underwent 3D-LLR at the laparoscopic center (Center 3) between 2015 and 2017. Of these, 49 patients had 3D-LLR for HCC. Before 2015, more than 300 cases were performed using the 2D laparoscopic approach at the laparoscopic center.
The two groups were similar in terms of the presence and severity of underlying liver disease and tumor burden. Pringle maneuver was more frequently used in the 3D-LLR group, and blood transfusion was more frequent in the RLR group (Table 2). Beyond the debate on the impact of clamping [23,24,25] and transfusion [26,27,28] on short- and long-term outcomes, the difference in transfusion observed herein was a consequence of the policies of different centers on the use of the Pringle maneuver. With similar indications for clamping, it is reasonable to hypothesize that the difference in transfusion would vanish, as both approaches are essentially the same (i.e., laparoscopic). Second, the higher proportion of major hepatectomy in the 3D-LLR group (almost twice the number of major hepatectomy in the RLR group) might partly explain the higher rate of Pringle maneuver in the 3D-LLR group [29]. Third, the rate of major hepatectomy in the robotic group was probably biased by a learning curve effect, which is probably not the case in the 3D laparoscopic group: this might lead to longer operative time, possibly increasing the blood loss. Finally, it is well known that the use of bipolar crush clamping technique in the robotic approach may increase bleeding, whereas the parenchymal transection is performed by the CUSA in the 3D laparoscopic approach: applying more liberally the Pringle maneuver in the laparoscopic group may explain why Pringle maneuver was more frequently used in the 3D laparoscopic group, and blood transfusion was more frequent in the robotic group.
Despite differences in clamping and transfusion, the two techniques achieved a nil mortality and similar morbidity, which is in accordance with the available knowledge.
Despite the differences in clamping and transfusion, the surgical margin width was also similar between the two groups, including surgical width of tumors located in the posterosuperior segments of the liver (segments VII and VIII), which are considered to be more difficult to safely access and resect [30,31,32,33].
The high OS rate (> 80% at 3 years) observed in the present study may be explained by the selection of patients with low tumor burdens (> 80% of BCLC stage A in each group) and noncirrhotic livers (> 37% in each group). Similar OS and RFS between the two groups were observed in this study. In addition to the issue of patient selection, this finding may be partly explained by the mean follow-up period, which was limited to 29 months. Although not statistically significant, the RFS at 3 years was 48% in the robotic group and 24% in the laparoscopic group. The short follow-up period (26 months) and the sample size limit might explain this difference.
Finally, from a technical point of view, although a 3D vision is shared between the two techniques, several points differ between the 3D vision laparoscopic and robotic surgery. Good vision is important not only for the surgeon but also for the assistants [34]. For the 3D laparoscopic procedures, the assistants used 3D polarized glasses for surgery (Fig. 1A). For the robotic procedures, unlike the primary surgeon, who uses the 3D visual console of the robot, the surgical assistants still have to depend on 2D images projected onto a flat-screen monitor (Fig. 1B). As the use of 3D vision improves the efficiency of the assistants in the setting of robotic urologic surgery, further studies are needed to assess the benefits of its routine use for assistants of robotic liver surgery. Yet, compared to rigid conventional 0 or 30° 3D optics, the use of a flexible 3D endoscope in LLR allows to expose vessels in any position (right, left, up, and down). The flexible endoscope allowed the alignment and maintenance of the vision and transection plane. In a previous study, we demonstrated the benefit of flexible 3D in right hepatectomy [6]. Finally, a main difference between the two procedures was the systematic use of CUSA in the LLR group, which is not yet available in robotic surgery. Although difficult to demonstrate, sharp dissection associated with pedicle clamping and 3D vision likely contributes to the low bleeding and transfusion rates we observed in the LLR series. Finally, the obvious lack of ergonomic in LLR compared to robotic surgery has probably been overcome owing to excellent vision and the transection technique.
We acknowledge the obvious limits of the present analysis. First, as in any retrospective study, patient selection bias cannot be ruled out. Second, the potential type 2 errors due to sample size could not be obviated, as in any preliminary study. Third, no cost analysis was performed in the present study. With regard to the costs of 3D technology, 3D laparoscopy is more affordable than robotic systems. However, previous studies have shown that whereas perioperative costs (due to robotic instruments) were significantly higher for the robotic procedure, total costs, including peri- and postoperative costs (i.e., the costs of the stay), were similar between the two procedures [8]. Fourth, no evaluation or comparison of the visualization between 2D and 3D surgical assistants was performed in this study. Fifth, another important point may be the fact that all cases were collected with different time frame (2011–2017 for the robotic group vs. 2015–2017 for the 3D laparoscopic group). This point represents an inherent bias of the comparison of two different technologies: the robotic approach was first performed in 2011 and the 3D laparoscopic approach was first performed in November 2014. Finally, there are substantial differences in the surgical technique that may have an impact on outcomes: (1) Pringle maneuver was done in 65% of cases in laparoscopy vs. 20% in robotic surgery, (2) the technique of parenchymal transection was different (CUSA for laparoscopic cases vs. Harmonic/Bipolar for robotic cases), and (3) the proportions of major and minor hepatectomies were different: almost the double of major resections in the laparoscopic group.
This multicenter comparative analysis suggests that 3D-LLR and RLR achieve similar operative times and short-term outcomes and similar safe margins and oncological long-term outcomes. This study shows the efficacy of using 3D vision during minimally invasive LR and provides a basis for recommending the 3D system in future prospective observational trials comparing laparoscopic and robotic approaches for liver cancer resection.
References
EASL Clinical Practice Guidelines (2018) Management of hepatocellular carcinoma. J Hepatol 69(1):182–236
Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, Asbun H, O’Rourke N, Tanabe M, Koffron AJ, Tsung A, Soubrane O, Machado MA, Gayet B, Troisi RI, Pessaux P, Van Dam RM, Scatton O, Abu Hilal M, Belli G, Kwon CH, Edwin B, Choi GH, Aldrighetti LA, Cai X, Cleary S, Chen KH, Schön MR, Sugioka A, Tang CN, Herman P, Pekolj J, Chen XP, Dagher I, Jarnagin W, Yamamoto M, Strong R, Jagannath P, Lo CM, Clavien PA, Kokudo N, Barkun J (2015) Strasberg SM (2015) Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 261(4):619–629
Buell JF, Cherqui D, Geller DA, O'Rourke N, Iannitti D, Dagher I, Koffron AJ, Thomas M, Gayet B, Han HS, Wakabayashi G, Belli G, Kaneko H, Ker CG, Scatton O, Laurent A, Abdalla EK, Chaudhury P, Dutson E, Gamblin C, D'Angelica M, Nagorney D, Testa G, Labow D, Manas D, Poon RT, Nelson H, Martin R, Clary B, Pinson WC, Martinie J, Vauthey JN, Goldstein R, Roayaie S, Barlet D, Espat J, Abecassis M, Rees M, Fong Y, McMasters KM, Broelsch C, Busuttil R, Belghiti J, Strasberg S, Chari RS (2009) The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 250(5):825–830
Liu R, Wakabayashi G, Kim HJ, Choi GH, Yiengpruksawan A, Fong Y, He J, Boggi U, Troisi RI, Efanov M, Azoulay D, Panaro F, Pessaux P, Wang XY, Zhu JY, Zhang SG, Sun CD, Wu Z, Tao KS, Yang KH, Fan J, Chen XP (2019) International consensus statement on robotic hepatectomy surgery in 2018. World J Gastroenterol 25(12):1432–1444
Kawai T, Goumard C, Jeune F, Savier E, Vaillant JC, Scatton O (2018) Laparoscopic liver resection for colorectal liver metastasis patients allows patients to start adjuvant chemotherapy without delay: a propensity score analysis. Surg Endosc 32(7):3273–3281
Kawai T, Goumard C, Jeune F, Komatsu S, Soubrane O, Scatton O (2018) 3D vision and maintenance of stable pneumoperitoneum: a new step in the development of laparoscopic right hepatectomy. Surg Endosc 32(8):3706–3712
Lim C, Salloum C, Tudisco A, Ricci C, Osseis M, Napoli N, Lahat E, Boggi U, Azoulay D (2019) Short- and long-term outcomes after robotic and laparoscopic liver resection for malignancies: a propensity score-matched study. World J Surg 43(6):1594–1603
Salloum C, Lim C, Lahat E, Gavara CG, Levesque E, Compagnon P, Azoulay D (2016) Robotic-assisted versus laparoscopic left lateral sectionectomy: analysis of surgical outcomes and costs by a Propensity Score Matched Cohort Study. World J Surg 41(2):516–524
Salloum C, Subar D, Memeo R, Tayar C, Laurent A, Malek A, Azoulay D (2014) Laparoscopic robotic liver surgery: the Henri Mondor initial experience of 20 cases. J Robot Surg 8(2):119–124
Salloum C, Lim C, Azoulay D (2015) Robot-assisted laparoscopic left lateral sectionectomy for benign and malignant liver tumors. J Visc Surg. https://doi.org/10.1016/j.jviscsurg.2015.09.07
Salloum C, Subar D, Memeo R, Tayar C, Laurent A, Malek A, Azoulay D (2013) Laparoscopic robotic liver surgery: the Henri Mondot initial experience of 20 cases. J Robotic Surg. https://doi.org/10.1007/s11701-013-0437-9
Spampinato MG, Coratti A, Bianco L, Caniglia F, Laurenzi A, Puleo F, Ettorre GM, Boggi U (2014) Perioperative outcomes of laparoscopic and robot-assisted major hepatectomies: an Italian multi-institutional comparative study. Surg Endosc 28(10):2973–2979
Boggi U, Caniglia F, Vistoli F, Costa F, Pieroni E, Perrone VG (2015) Laparoscopic robot-assisted resection of tumors located in posterosuperior liver segments. Updates Surg 67(2):177–183
Boggi U, Caniglia F, Amorese G (2013) Laparoscopic robot-assisted major hepatectomy. J Hepatobiliary Pancreat Sci 21(1):3–10
Bismuth H (1982) Surgical anatomy and anatomical surgery of the liver. World J Surg 6(1):3–9
Lim C, Salloum C, Lahat E, Sotirov D, Eshkenazy R, Shwaartz C, Azoulay D (2019) Impact of narrow margin and R1 resection for hepatocellular carcinoma on the salvage liver transplantation strategy. An intention-to-treat analysis. HPB, Oxford
Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, Fan ST, Yokoyama Y, Crawford M, Makuuchi M, Christophi C, Banting S, Brooke-Smith M, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Nimura Y, Figueras J, DeMatteo RP, Büchler MW, Weitz J (2011) Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 149(5):680–688
Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, Banting S, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Yokoyama Y, Fan ST, Nimura Y, Figueras J, Capussotti L, Büchler MW, Weitz J (2011) Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 149(5):713–724
Rahbari NN, Garden OJ, Padbury R, Maddern G, Koch M, Hugh TJ, Fan ST, Nimura Y, Figueras J, Vauthey JN, Rees M, Adam R, Dematteo RP, Greig P, Usatoff V, Banting S, Nagino M, Capussotti L, Yokoyama Y, Brooke-Smith M, Crawford M, Christophi C, Makuuchi M, Büchler MW, Weitz J (2011) Post-hepatectomy haemorrhage: a definition and grading by the International Study Group of Liver Surgery (ISGLS). HPB (Oxford) 13(8):528–535
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA (2013) The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 258(1):1–7
Benchimol EI, Langan S, Guttmann A (2012) Call to RECORD: the need for complete reporting of research using routinely collected health data. J Clin Epidemiol 66(7):703–705
Famularo S, Giani A, Di Sandro S, Sandini M, Giacomoni A, Pinotti E, Lauterio A, Gianotti L, De Carlis L, Romano F (2017) Does the Pringle maneuver affect survival and recurrence following surgical resection for hepatocellular carcinoma? A western series of 441 patients. J Surg Oncol 117(2):198–206
Xu W, Xu H, Yang H, Liao W, Ge P, Ren J, Sang X, Lu X, Zhong S, Mao Y (2016) Continuous Pringle maneuver does not affect outcomes of patients with hepatocellular carcinoma after curative resection. Asia Pac J Clin Oncol 13(5):e321–e330
Liu S, Li X, Li H, Guo L, Zhang B, Gong Z, Zhang J, Ye Q (2016) Longer duration of the Pringle maneuver is associated with hepatocellular carcinoma recurrence following curative resection. J Surg Oncol 114(1):112–118
Yamashita YI, Hayashi H, Imai K, Okabe H, Nakagawa S, Kitamura F, Uemura N, Nakao Y, Yusa T, Itoyama R, Yamao T, Umesaki N, Miyata T, Chikamoto A, Shimokawa M, Baba H (2019) Perioperative allogeneic blood transfusion does not influence patient survival after hepatectomy for hepatocellular carcinoma: a propensity score matching analysis. World J Surg 43(11):2894–2901
Peng T, Zhao G, Wang L, Wu J, Cui H, Liang Y, Zhou R, Liu Z, Wang Q (2017) No impact of perioperative blood transfusion on prognosis after curative resection for hepatocellular carcinoma: a propensity score matching analysis. Clin Transl Oncol 20(6):719–728
Yang T, Lu JH, Lau WY, Zhang TY, Zhang H, Shen YN, Alshebeeb K, Wu MC, Schwartz M, Shen F (2015) Perioperative blood transfusion does not influence recurrence-free and overall survivals after curative resection for hepatocellular carcinoma: a propensity score matching analysis. J Hepatol 64(3):583–593
Fiorentini G, Swaid F, Cipriani F, Ratti F, Heres C, Tsung A, Aldrighetti L, Geller DA (2019) Propensity score-matched analysis of pure laparoscopic versus hand-assisted/hybrid major hepatectomy at two western centers. World J Surg 43(8):2025–2037
Cho JY, Han HS, Yoon YS, Shin SH (2008) Feasibility of laparoscopic liver resection for tumors located in the posterosuperior segments of the liver, with a special reference to overcoming current limitations on tumor location. Surgery 144(1):32–38
Patriti A, Cipriani F, Ratti F, Bartoli A, Ceccarelli G, Casciola L, Aldrighetti L (2014) Robot-assisted versus open liver resection in the right posterior section. JSLS 18(3):e2014
Aghayan DL, Fretland ÅA, Kazaryan AM, Sahakyan MA, Dagenborg VJ, Bjørnbeth BA, Flatmark K, Kristiansen R, Edwin B (2019) Laparoscopic versus open liver resection in the posterosuperior segments: a sub-group analysis from the OSLO-COMET randomized controlled trial. HPB, Oxford
Montalti R, Scuderi V, Patriti A, Vivarelli M, Troisi RI (2015) Robotic versus laparoscopic resections of posterosuperior segments of the liver: a propensity score-matched comparison. Surg Endosc 30(3):1004–1013
Ramanathan R, Salamanca JI, Mandhani A, Leung RA, Rao SR, Berryhill R, Tewari A (2009) Does 3-dimensional (3-D) visualization improve the quality of assistance during robotic radical prostatectomy? World J Urol 27(1):95–99
Funding
The authors do not receive any funding from Intuitive or 3D laparoscopic system.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Chetana Lim, Dr. Claire Goumard, Dr. Chady Salloum, Dr. Antonella Tudisco, Dr. Niccolo Napoli, Prof. Ugo Boggi, Prof. Daniel Azoulay, and Prof. Olivier Scatton have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lim, C., Goumard, C., Salloum, C. et al. Outcomes after 3D laparoscopic and robotic liver resection for hepatocellular carcinoma: a multicenter comparative study. Surg Endosc 35, 3258–3266 (2021). https://doi.org/10.1007/s00464-020-07762-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-07762-7