Abstract
Introduction
Nowadays in Europe, laparoscopic ventral mesh rectopexy is the gold standard treatment of external rectal prolapse (ERP). The benefits of robot ventral mesh rectopexy (RVMR) are not clearly defined. The primary objective of the study was to evaluate the long-term results of RVMR. The secondary objective was to determine predictive factors of recurrence.
Design
Monocentric, retrospective study. Data, both pre-operative and peri-operative, were collected, and follow-up data were assessed prospectively by a telephone questionnaire. The study was performed in a tertiary referral center.
Methods
Between August 2007 and August 2017, we evaluate all consecutive patients who underwent RVMR for ERP by three different surgeons. The primary outcome was the recurrence rate perceived by patients. Secondary outcome were functional results based on Knowles–Eccersley–Scott-Symptom score for constipation and Wexner score for incontinence, compared before and after surgery.
Results
During the study period 96 patients (86 women) underwent RVMR. The mean age was 62.3 years (range 16–90). Twelve patients had a history of ERP repair. Sixty-nine patients were analyzed for long-term outcomes with a mean follow-up of 37 months (range 2.3–92 months). Recurrence rate was 12.5%. After surgery, constipation was significantly reduced: 44 patients were constipated before surgery versus 23 after surgery. Six patients described de novo constipation (6.25%). Fecal incontinence was significantly reduced: 59 patients were incontinent before surgery versus 14 after surgery. No predictive factor for recurrence was identified after multivariate analysis. No mesh related complications were related.
Conclusions
In conclusion, RVMR presents good long-term functional result and a recurrence rate similar to LVMR as published in the literature. The rate of mesh related complications seems lower.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
External rectal prolapse (ERP) is a common and disabling pathology. ERP is often associated with functional disorders: constipation and fecal incontinence [1, 2]. Many surgical techniques have been described to treat ERP. The literature suggests that abdominal rectopexy procedures offer the best prospects for cure, with a lower recurrence rate compared to a perineal approach [3,4,5]. Laparoscopic approach was compared to open surgery, and many studies have concluded that it is superior in terms of post-operative pain, post-operative ileus, length of hospital stay [6], and economic impact [7]. Laparoscopic approach is nowadays the gold standard approach for abdominal rectopexy. Till recently, most abdominal rectopexy procedures included a posterior dissection of the rectum and were followed by a high rate of de novo constipation [8, 9]. D’Hoore et al. described a single mesh ventral rectopexy technique without any posterolateral rectal mobilization, minimizing the risk of post-operative constipation [10] which was confirmed by other authors [11,12,13]. D’Hoore et al. have reported satisfying long-term outcomes in terms of recurrence [14]. In Europe, this procedure is gradually becoming the gold standard to treat ERP [15,16,17,18,19,20]. At the beginning of the twenty first century, robotic assistance started being used in general surgery [21]. In 2004 Munz et al. reported the first experience of robotic rectopexy for ERP [22], and we reported our first results in 2004 [21].
Due to its technical advantages, we thought that the robotic approach could be profitable for both the patient and the surgeon.
We demonstrated in 2013 that the learning curve of the robotic approach for ERP was shorter than the learning curve of the laparoscopic approach with similar anatomic and functional long-term results. The major limitation of this study was the heterogeneity of the surgical procedures used, including Orr–Loygue, Frikman Golberg and D’Hoore ‘s techniques [23]. Concerning robotic ventral mesh rectopexy (RVMR), there is no study in the literature showing the superiority of the robotic approach compared to the laparoscopic approach [24,25,26,27]. Furthermore, previous robotic or laparoscopic series included ERP, rectocele, and internal prolapse.
The aim of our study was to report the long-term outcomes of RVMR for ERP only, performed in our center.
Methods
Trial design
Demographic, peri-operative data and short-term outcome data at 6 weeks were collected retrospectively. Long-term outcomes were assessed prospectively by a standardized telephone questionnaire.
This study was approved by our Institutional Review Board.
Participants
We searched for the files of patients who underwent RVMR for ERP from August 2007 to August 2017 in a database using the CCAM code. Only patients who underwent RVMR were included. Between 2007 to March 2009 we still performed Orr–Loygue’s procedures but we progressively switched to D’Hoore procedure and since May 2009, exclusively D’Hoore procedure was performed to treat ERP. All patients with full thickness rectal prolapse were operated whatever independently of the functional pre-operative symptoms, so they were no selection of patient for surgery.
Surgical procedure
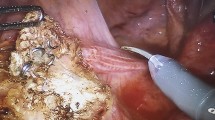
All procedures were performed by three experienced surgeons, with a Da Vinci robotic system Intuitive Surgical, Sunnyvale, CA. (standard from 2007 to 2012 and Si since 2012). Since the beginning, the procedure was standardized, and there was no technical modification during the analysis period. We never performed a laparoscopic approach for rectopexy in our center. A rectal enema was performed the day before and the morning before surgery. The patient was positioned in Trendelenburg position. The robot was placed between the patient’s legs. We used 5 ports, including a camera port of 12 mm just below the umbilicus, an 8 mm port in the right iliac fossa for the right robotic arm, an 8 mm port in the left iliac fossa for the left robotic arm, and 2 laparoscopic ports, one measuring 10 mm and the other measuring 5 mm for the assistants on the right and left flanks. Dissection started at the sacral promontory with preservation of the right hypogastric nerve. The incision was extended caudally in an inverted J shape along the rectum and over the deepest part of the pouch of Douglas. The rectovaginal septum was opened down to the pelvic floor. A non-absorbable mesh was inserted (Prolene 4 cm width × 17 cm long). The mesh was sutured to the ventral aspect of the distal rectum with nine 3/0 braided non-absorbable stitches. In case of associated genital prolapse, we included the posterior vaginal wall in the suture as described by D’Hoore et al. [10] and we added an anterior mesh to treat the cystocele. The mesh was then fixed to the sacral promontory without tension with two 3/0 braided non-absorbable stitches. A peritoneal closure was always performed at the end of surgery with an absorbable running suture (Fig. 1).
Outcomes
Our first objective was to evaluate the rate of recurrence after RVMR. Recurrence was defined as full or partial thickness prolapse perceived by the patients. Mucosal prolapses were not considered as a recurrence after examination by a surgeon.
Our second objective was to evaluate long-term functional results after RVMR. Constipation based on Kess score (Knowles–Eccersley–Scott-Symptom) [28] and fecal incontinence based on Wexner score [29] were evaluated and compared to the pre-operative data. A constipation was defined by a Kess score > 10, and a fecal incontinence was defined by a Wexner score > 7.
We collected peri-operative data: age, sex, pre-operative constipation and pre-operative fecal incontinence using dedicated questionnaires, history of pelvi-perineal surgery (previous abdominal rectopexy, Delorme or Altemeier procedure, hysterectomy, urogenital prolapse surgery), operating time (from incision to wound closure, including docking and undocking of the robot), conversion to open procedure, associated bladder prolapse treatment, post-operative complications, and length of hospital stay.
At 6 weeks after surgery, all patients had a follow-up consultation with the surgeon. We collected the following data: recurrence, constipation, and fecal incontinence. A clinical examination was performed by the surgeon, to look for a full or partial thickness prolapse or for a mucosal prolapse. Kess score and Wexner score were completed for each patient.
At long-term follow-up, we evaluated prospectively the following data with a standardized telephone questionnaire: recurrence, constipation (based on Kess score), fecal incontinence (based on Wexner score), and global satisfaction on a 5-point scale. In case of perceived recurrence by the patient, a surgical consultation was schedule.
Statistical analysis
Data were analyzed with the use of SAS 9.2 (SAS Institute, Inc., Cary, NC) for actuarial recurrence rate using Kaplan–Meier method. This method was the most relevant because of the large range of follow-up time (2.3 to 92 months). Microsoft Excel 2017 (Microsoft Office, Microsoft, Redmond, WA) was used to produce all of the other statistical data. Statistical tests were performed on predictive factor for recurrence, using Cox model to realize bivariate and multivariate analysis.
Results
Demographic data
Ninety-six patients were treated for ERP by RVMR between August 2007 and August 2017. Sixty-nine (71.9%) of them were eligible for long-term follow-up and completed the telephone questionnaire. Median follow-up time was 31 months, and mean follow-up time was 37 months (range 2.3–92 months). Seven patients died of unrelated causes during follow-up. Demographic data are summarized in Table 1 and flow chart (Fig. 2).
Twelve patients had a history of ERP repair: 4 perineal repairs by Delorme’s procedure and 8 Orr–Loygue’s procedure.
Before surgery, 59 patients (61.5%) presented fecal incontinence, with a mean Wexner score of 9.0/20 (range 0–20), and 44 patients (45.8%) suffered from constipation with a mean Kess score of 7.0/39 (range 0–28).
Intra-operative and post-operative data
Five patients (5.20%) needed a conversion to laparotomy; three for dissection difficulties in patients who had undergone previous abdominal colpopexy and two for bleeding during promontory dissection (Table 2).
There was no mortality. Complications were classified according to Clavien–Dindo [30]. Morbidity was noted in 12 patients (12.5%), 1 Clavien IV, 6 Clavien III, 1 Clavien II, and 4 Clavien I (Table 3).
Short-term follow-up: 6 weeks after surgery
Recurrence
One patient presented with early recurrence (1.04%).
Functional results
The fecal incontinence rate was significantly improved: 16.7% (16 patients) versus 61.5% (59 patients) before surgery. Mean Wexner score before surgery was 9.0/20 (range 0–20), and mean score after surgery was 1.0/20 (range 1–18, p < 0.0001).
The constipation rate decreased significantly: 30% (29 patients) had a Kess score superior to 10 after surgery versus 45.8% (44 patients) before surgery. Mean Kess score before surgery was 7.0/39 (range 0–28), and mean score after surgery was 5.0 (range 0–28, p = 0.01).
Five patients (5.2%) reported de novo constipation.
Long-term outcome
Sixty-nine patients (71.9%) were eligible for follow-up after a mean time of 30 months (range 3–93 months). Lost population was similar to followed population (Table 4). Among the 27 patients lost to follow-up: 7 were dead, 6 had given a wrong telephone number, and 14 didn’t reply to our repeated phone calls (Fig. 3).
Recurrence
Twelve patients (12.5%) perceived recurrence at long-term follow-up. Eleven of them underwent subsequent surgery and two of them had a secondary recurrence. One patient declined surgery (Fig. 4).
Functional results
The fecal incontinence rate was improved: 14.6% (14 patients) versus 61.5% (59 patients) before surgery. Mean Wexner score before surgery was 9.0/20 (range 0–20) and mean Wexner score after surgery was 1.0/20 (range 0–18), p < 0.0001 (IC95%) (Fig. 5).
The constipation rate was improved: 23.9% (23 patients) had a Kess score superior to 10 after surgery versus 45.8% (44 patients) before surgery. Mean score before surgery was 7.0 (range 0–28) and mean score after surgery was 6.0 (range 0–26), p = 0.01 (IC95%). Six patients (6.25%) were diagnosed with de novo constipation (Fig. 6).
On average, patients were either satisfied (33.82%) or very satisfied (35.29%) with their surgery. Four patients were dissatisfied (5.8%).
Predictive factors for recurrence
No predictive factor for recurrence was identified with double and multivariate analysis (Table 5). Only a history of perineal surgery seemed to be a risk of recurrence with HR of 7.38 but it was not significant p = 0.06 in bivariate and p = 0.65 multivariate analysis.
Long-term complications
No mesh infection or mesh erosion was observed during the follow-up period.
Discussion
Summary of our results
To the best of our knowledge this study is the first study of RVMR for ERP with long-term follow-up. At a mean follow-up of 3 years, the recurrence rate was 12.5%. Fecal incontinence rate decreased significantly, and the mean post-operative Wexner score changed from 9.0 to 1.0. The constipation rate was improved: 23.9% (23 patients) had a Kess score superior to 10 after surgery versus 45.8% (44 patients) before surgery. Previous perineal surgery increased the recurrence risk HR = 7.38 (p = 0.06, non-significant). The rate of de novo constipation was 6.25%. There was no mesh infection or mesh erosion during the follow-up period.
Recurrence
Our recurrence rate was 12.5%, with a mean follow-up of 3 years. Recurrence rate in our cohort is comparable with the recurrence rate in recent studies including robotic and laparoscopic approach [15, 18, 24]. In 2015, Consten et al., in a multi-centric study of 242 patients treated for ERP reported an actuarial recurrence rate of 8.2% after 10 years with LVMR [15]. In 2016, Van Iersel et al. reported on a series of 48 RVMR for ERP with an actuarial recurrence rate of 12.9% after 5 years [25]. Concerning the early recurrences, Badrek-Al Amoudi et al. operated eight patients with early recurrences. In seven cases the recurrence was related to the detachment of the mesh from the promontory or incorrectly positioned staples on the upper sacrum [31]. In our series, we have only one early recurrence and this good result is maybe related to the use of suture of the mesh to the rectal wall and to the promontory by stitches, which is very simple to accomplish with the robotic approach. Cherylin et al. have reported a high recurrence rate for ERP with LVMR: 22.1% (25/113). This poor result could be explained by a broad definition of recurrence including total rectal prolapse and mucosal prolapse [32].
In our series, half of the patients who had recurrences had been previously operated for total rectal prolapse. Our results suggest that previous rectal prolapse surgery could increase the recurrence rate. Gurland et al. showed that patients who had undergone prior prolapse surgery had a higher risk of prolapse recurrence, 25% versus 6.9%. Time to recurrence was significantly shorter for patients with prior rectal prolapse surgery in her series (8.8 vs. 30.7 months, p = 0.03) [33].
In our series, no clear risk factor for recurrence was identified. There are few data in the literature about risk factors for recurrence. Cherylin et al. in a multi-centric series including 113 patients suffering from ERP, found risk factors for recurrence including age > 70 years (HR = 2.22, p = 0.005), worse pre-operative Cleveland clinic incontinence score (HR = 1.18, p = 0.002), prolonged pudendal nerve terminal motor latency (HR = 6.69, p = 0.001), and the use of synthetic mesh versus biological mesh (HR = 2.71, p = 0.002) [32]. In contradiction Badrek-Al Amoudi et al. reported 63.3% (7/11) cases of recurrence in patients treated with Permacol mesh [31].
Functional results
Our long-term functional results of RVMR are comparable to the results of LVMR and RVMR series.
Consten et al. reported 63.4% of fecal incontinence improvement in LVMR for ERP in 242 patients [15]. Van Iersel et al. reported 76.9% improvement in fecal incontinence after RVMR in 48 patients [25]. We reported an improvement of 69.56% in fecal incontinence. Consten et al. reported a 61.0% improvement in constipation symptoms after LVMR [15], Van Iersel et al. reported 84.9% improvement in constipation symptoms after RVMR [25]. In our study there was an improvement of 57.97% in constipation symptoms. Greater improvement in obstructed defecation after RVMR was observed by Mantoo et al. who performed both LVMR and RVMR (ODS score 23 vs. 18 after LVMR and 22 vs. 13 after RVMR, p = 0.004) [34]. The reason for the improvement in RVMR is possibly due to the technical advantages associated with robotic assistance. These might include improved autonomic nerve-sparing (the anterior-only rectal mobilization minimizing the chance of lateral damage to the pelvic nerves) a deeper mesh placement and a greater reduction of the rectocele. Precise suturing of mesh to the pelvic floor muscles may also improve the mesh placement and pelvic floor support during RVMR.
Concerning the de novo constipation rate after ventral rectopexy, two meta-analyses demonstrated, that ventral mesh rectopexy seems to induce less de novo constipation as compared to other abdominal techniques of rectopexy [4, 9]. We reported in our series of RVMR a 6.25% de novo constipation rate. In a previous study published in 2013, we reported a 24% de novo constipation rate with robotic rectopexy, but most of the procedures included a posterior dissection of the rectum [23]. These data clearly confirm that RVMR is superior to the procedure including posterior dissection in terms of de novo constipation [35]. A recent meta-analysis studying ventral mesh rectopexy only, reported a de novo constipation rate between 0 and 20% [20].
Long-term complications
We report a 5.2% of incisional hernia, this can be compared to the rate of 2.2% reported by Van Iersen et al. [25]. All incisional hernia appeared on the 12 mm port site although we have always closed the muscle and aponevrosis with absorbable stitches. We never observed incisional hernia on the 8 mm port site. One can imagine that the use of the new generation of Da Vinci robot which includes only 8 mm ports will decrease the incidence of incisional hernia.
In our series after 3 years of follow-up, there was no related mesh complication. The use of surgical mesh in the repair of rectal prolapse has, however, raised concerns. In 2015, in a multi-centric and international series, Evans et al. carried out a retrospective review reporting mesh morbidity in a large group of 2203 patients. Two percent developed mesh erosion after LVMR [18].
Different factors responsible for mesh complications have been discussed in the literature: type of mesh, mesh size, and method of fixation. Smart et al. in their meta-analysis, found no significant difference between synthetic and biological mesh (0.7% vs. 0%, p = 1.0%) [36]. However, in this review there was a difference of length of median follow between the synthetic mesh group (up to 74 months) and the biological mesh group (12 months). It leads us to suppose that with longer follow-up, more recurrence will become evident in patients with biological mesh. For Evans et al., mesh erosion has been related to the pore size of the material used (pore size > 75 μm could reduce risk) [18].
Concerning the method of fixation of the mesh, there are no randomized analyses which evaluate the difference between stitches, tacks, staples, or glue. RVMR using stitches to fix the mesh to the rectum and the sacral promontory seems to have low mesh related complications as reported by Van Iersel in 2017 (1.3%) [25]. Our results confirm a trend towards a reduction of post-operative mesh complications in RVMR. This good result could be related to better intra-corporeal suturing performance, and to better mesh positioning due to robotic assistance [36]. Van Iersel reported 1.3% of mesh complication with RVMR (one case of mesh erosion on the posterior wall of the vagina) [25] versus 4.6% with LVMR (at 10 years of follow-up). Consten et al. reported 9 cases of mesh detachment, 7 cases of mesh erosion, 1 case of obstruction and pre-sacral adhesions mesh, and 1 case of chronic mesh infection and fistula after LVMR in 790 patients [15].
The low rate of mesh related complications could be a clear economic advantage of robotic surgery compared to the laparoscopic approach.
Cost
The cost of RVMR is still a major drawback for robotic assistance. Heemskerk et al. compared LVMR and RVMR for ERP and quoted a difference of 557.29€ (or 745.09$ p = 0.01) in favor of LVMR, which is clearly understatement because the authors do not include the purchase and maintenance of the robot. The higher cost of the robotic approach cannot be justified yet without evidence for the superiority of RVMR [37]. However, as the use of robotics in colorectal and other surgical fields becomes more wide-ranging, this may subsequently reduce cost implications, leading to greater acceptance of the technique [38]. Moreover, very low morbidity could help to reduce the overall cost of robotic assistance. Further comparative studies are needed to evaluate the cost-effectiveness of this approach.
Limitations
Our study has some limitations. It was a non-comparative case series from a single institution. The loss of patients to follow-up was notable, but the lost population was similar to the followed population. A 71.9% response rate for the questionnaires should be considered acceptable, comparable to other studies. Makela-Kaikkonen et al.’s reported 29% of lost patients after 44 months’ follow-up [19], Consten et al. reported 14% of lost patients after 3 months of LVMR follow-up [15], Van Iersen et al. reported 12% of lost patients after 6 weeks of follow-up [25]. As in all these studies, our results are calculated on the population initially included. The high rate of loss population of follow-up, is a major bias in interpreting our results, and we may have underestimated our recurrence rate.
Our mean hospital stay is 4.0 days, but since 2014, it has gradually decreased to 3 days. The mean hospital stay has improved with experience. For the 20 first patients, the mean hospital stay was 6 days compared to 3 days for the last 20 patients. Nowadays, most of patients arrive the day before surgery and are able to go home on day 1 or day 2 after surgery for the older patients.
We will soon begin 1-day surgery procedures as described recently by Faucheron et al. for RVMR and LVMR [39].
Conclusion
RVMR is a good alternative to LVMR for ERP treatment. It offers similar outcomes in terms of recurrence, and good long-term functional results. RVMR could be superior to LVMR in terms of mesh erosions. RVMR offers many advantages for the surgeons including: easier dissection, improved dexterity for suturing in the narrow space of the pelvis, improved physiological and ergonomic comfort, and a short learning curve [23]. For all these reasons, in our team RVMR is the preferred approach for ERP repair.
References
D’Hoore A (2003) Obstructed defecation. Colorectal Dis 5:280–287
Bordeianou L, Hicks CW, Kaiser AM et al (2014) Rectal prolapse: an overview of clinical features, diagnosis and patients-specific management strategies. J Gastrointest Surg 18:1059–1069
Marchal F, Bresler L, Ayav A et al (2005) Long-term results of Delorme’s procedure and Orr–Loygue rectopexy to treat complete rectal prolapse. Dis Colon Rectum 48:1785–1790
Madiba TE, Baig MK, Wexner SD (2005) Surgical management of rectal prolapse. Arch Surg 140:63–73
Penninckx F, D’Hoore A, Sohier S, Kerremans R (1996) Abdominal rectopexy versus Delorme’s procedure: a predicable outcome. Int J Colorectal Dis 12:49–50
Senagore AJ (2003) Management of rectal prolapse: the role of laparoscopic approaches. Semin Laparosc Surg 10:197–202
Salkeld G, Bagia M, Solomon M (2004) Economic impact of laparoscopic versus open abdominal rectopexy. Br J Surg 91:1188–1191
Brown AJ, Anderson JH, McKee RF, Finlay IG (2004) Strategy for selection of operation for rectal prolapse based on clinical criteria. Dis Colon Rectum 47:103–107
Cadeddu F, Sileri P, Grande M, De Luca E, Franceschilli L, Milito G (2012) Focus on abdominal rectopexy for full thickness rectal prolapse: meta-analysis of literature. Tech Coloproctol 16:37–53
D’Hoore A, Cadoni R, Penninckx F (2004) Long term outcome of laparoscopic ventral rectopexy for total rectal prolapse. Br J Surg 91:1500–1505
Mollen RM, Kuipers JH, Van Hoek F (2000) Effects of rectal mobilization and lateral ligaments division on colonic anorectal function. Dis Colon Rectum 43:1283–1287
Scaglia M, Fasth S, Hallgren T, Nordgren S, Oresland T, Hulten L (1994) Abdominal rectopexy for rectal prolapse: influence of surgical technique on functional outcome. Dis Colon Rectum 37:805–813
Speakman CTM, Madden MV, Nicholls RJ, Kamm MA (1991) Lateral ligament division during rectopexy causes constipation but prevents recurrence: results of a prospective randomized study. Br J Surg 78:1431–1433
D’Hoore A, Penninckx F (2006) Laparoscopic ventral recto(colpo)pexy for rectal prolapse: surgical technique ant outcome for 109 patients. Surg Endosc 20:1919–1923
Consten E, Van Iersel J, Verheijen P, Broeders I, Wolthuis A, D’Hoore A (2015) Long-term outcome after laparoscopic ventral mesh rectopexy. Ann Surg 262:742–748
Van Iersel J, Paulides T, Verheijen P, Lumley J, Broeders I, Consten E (2016) Current status of laparoscopic and robotic ventral mesh rectopexy for external and internal rectal prolapse. World J Gastroenterol 22:4977–4987
Rogers C, McCawley N, Hanly M, Deasy J, Mc Namara A, Burke P (2018) Trends in treatment of rectal prolapse: population analysis. Int J Colorectal Dis 33:459–465
Evans C, Stevenson A, Sileri P, Mercer-Jones M, Dixon A, Cunningham C, Jones O, Lindsey I (2015) A multicenter collaboration to assess the safety of laparoscopic ventral rectopexy. Dis Colon Rectum 58:799–807
Makela-Kaikkonen J, Rautio T, Kairaluoma M, Carpelan-Holmstrom M, Kossi J, Rautio A, Ohtonen P, Makela J (2018) Does ventral rectopexy improve pelvic floor function in the long term? Dis Colon Rectum 61:230–238
Faucheron JL, Trilling B, Girard E, Sage PY, Barbois S, Reche F (2015) Anterior rectopexy for full-thickness rectal prolapse: technical and functional results. World J Gastroenterol 21:5049–5055
Ayav A, Bresler L, Brunaud L, Boissel P (2004) Early results of one-year robotic surgery using the Da Vinci system to perform advanced laparoscopic procedures. J Gastrointest Surg 8:720–726
Munz Y, Moorthy K, Kudchadkar R, Hernandez JD, Martin S, Darzi A, Rockall T (2004) Robotic assisted rectopexy. Am J Surg 187:88–92
Perrenot C, Germain A, Scherrer ML, Ayav A, Brunaud L, Bresler L (2013) Long-term outcomes of robot assisted laparoscopic rectopexy for rectal prolapse. Dis Colon Rectum 56:909–914
Inaba S, Sujatha-Bhaskar S, Koh Y, Jafari D, Milss D, Carmichael C, Stamos J, Pigazzi A (2017) Robotic ventral mesh rectopexy for rectal prolapse: a single institution experience. Tech Coloproctol 21:667–671
Van Iersel J, Formijne Jonkers H, Paulides T, Verheijen P, Draaisma W, Consten E, Broeders I (2017) Robot-assisted ventral mesh rectopexy for rectal prolapse: a 5-year experience at tertiary referral center. Dis Colon Rectum 60:1215–1223
Makela-Kaikkonen J, Rautio T, Koivurova S, Paakko E, Ohtonen P, Biancari F, Makela J (2016) Anatomical and functional changes to the pelvic floor after robotic versus laparoscopic ventral rectopexy: a randomized study. Int Urogynecol J 27:1837–1845
Ramage L, Georgio P, Tekkis P, Tan E (2015) Is robotic ventral mesh rectopexy better than laparoscopy in the treatment of rectal prolapse and obstructed defecation? A meta-analysis. Tech Coloproctol 19:381–389
Knowles CH, Scott SM, Legg PE, Allison ME, Lunniss PJ (2002) Level of classification performance of KESS (syndrome scoring system for constipation) validated in a prospective series of 105 patients. Dis Colon Rectum 45:842–843
Seong MK, Jung S, Kim TW, Joh HK (2011) Comparative analysis of summary scoring systems in measuring fecal incontinence. J Korean Surg 81:326–331
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336, patients and results of a survey. Ann Surg 240:205–213
Badrek-Al Amoudi AH, Greenslade GL, Dixon AR (2013) How to deal with complications after laparoscopic ventral mesh rectopexy: lessons learnt from a tertiary referral centre. Colorectal Dis 15:707–712
Cherylin W, Andrew R, Stevenson M (2017) Risk factors for recurrence after laparoscopic ventral rectopexy. Dis Colon Rectum 60:178–186
Gurland B, Carvalho ME, Ridgeway B, Paraiso MF, Hull T, Zutshi M (2017) Should we offer ventral rextopexy to patients with recurrent external rectal prolapse? J Colorectal Dis 32:1561–1567
Mantoo S, Podevin J, Regenet N, Rigaud J, Lehur PA, Meurette G (2013) Is robotic-assisted ventral mesh rectopexy superior to laparoscopic ventral mesh rectopexy in the management of obstructed defecation? Colorectal Dis 15:469–475
Germain A, Perrenot C, Scherrer ML et al (2014) Long-term outcome of robotic-assisted laparoscopic rectopexy for full-thickness rectal prolapse in elderly patients. Colorectal Dis 16:198–202
Smart NJ, Pathak S, Boorman P, Daniels IR (2013) Synthetic or biological mesh use in laparoscopic ventral mesh rectopexy—a systematic review. Colorectal Dis 15:650–654
Heemskerk J, De Hoog DE, Van Germert WG, Baeten CG, Greve JW, Bouvy ND (2007) Robot assisted vs conventional laparoscopic rectopexy for rectal prolapse: a comparative study of costs and time. Dis Colon Rectum 50:1825–1830
Jensen CC, Madoff RD (2016) Value of robotic colorectal surgery. Br J Surg 103:12–13
Faucheron JL, Trilling B, Barbois S, Sage PY, Waroquet PA, Reche F (2016) Day case robotic ventral rectopexy compared with day case laparoscopic ventral rectopexy: a prospective study. Tech Coloproctol 20:665–700
Author information
Authors and Affiliations
Contributions
Agathe Postillon substantial contributions to the conception and design of the work; and the acquisition, analysis, and interpretation of data for the work; and drafting the work and revising it critically for important intellectual content; and final approval of the version to be published; and Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy and integrity of any part of the work are appropriately investigated and resolved. Cyril Perrenot drafting the work and revising it critically for important intellectual content. Adeline Germain substantial contributions to the conception and design of the work; and the acquisition, analysis, and interpretation of data for the work; and drafting the work and revising it critically for important intellectual content. Marie-Lorraine Scherrer substantial contributions to the conception and design of the work; and the acquisition of data for the work. Cyrille Buisset substantial contributions to the conception and design of the work; and the acquisition, analysis, and interpretation of data for the work; and drafting the work and revising it critically for important intellectual content; and final approval of the version to be published. Laurent Brunaud final approval of the version to be published. Ahmet Ayav final approval of the version to be published. Laurent Bresler substantial contributions to the conception and design of the work; and the acquisition, analysis, and interpretation of data for the work; and drafting the work and revising it critically for important intellectual content; and final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy and integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Disclosures
Laurent Bresler, MD, PhD has financial disclosure; he is Proctor for Intuitive Surgical (Sunnyvale, CA). Agathe Postillon, Cyrille Perrenot, MD, Adeline Germain, MD, PhD, Marie-Lorraine Scherrer, MD, Cyrille Buisset, MD, Laurent Brunaud, MD, PhD, and Ahmet Ayav, MD, PhD have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Postillon, A., Perrenot, C., Germain, A. et al. Long-term outcomes of robotic ventral mesh rectopexy for external rectal prolapse. Surg Endosc 34, 930–939 (2020). https://doi.org/10.1007/s00464-019-06851-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-06851-6