Abstract
Background
The recurrent laryngeal nerve (RLN) lymph nodes are among the most frequently involved lymph nodes in esophageal cancer. Surgical removal of these lymph nodes is considered beneficial for postoperative prognosis, especially in patients with squamous cell carcinoma. Unfortunately, the precise surgical anatomy of the upper mediastinum is not well understood and no distinct high-resolution images are currently available.
Methods
In this article, we provide a simple intuitive concept of upper mediastinal surgical anatomy that could facilitate rational anatomical lymphadenectomy of the RLN lymph nodes. The essential concept of this mesenteric excision is to mobilize mesoesophagus including RLN in an en bloc fashion and to save RLN laterally by incising visceral sheath. This is applicable identically to both right and left upper mediastinum.
Results
Between January 2009 and December 2017, thoracoscopic esophagectomy with upper mediastinal lymphadenectomy for primary esophageal cancer was performed in 189 patients. Median thoracoscopic procedure time was 297 (range 205–568) min and median intraoperative blood loss was 70 ml (range unmeasurable up to 2545 ml). Median number of harvested upper mediastinal lymph nodes was 12. Postoperative complication of Clavien–Dindo classification grade III or higher events was observed in 14% of patients. RLN palsy of grade II or higher occurred in 20 patients (11%).
Conclusion
The mesoesophagus in the upper mediastinum is an anatomical unit surrounded by fibrous connective tissue containing the esophagus, trachea, tracheoesophageal vessels, lymphatic tissue, and RLNs. Thus, mesenteric excision of esophagus is defined to resect this area by sparing trachea and RLNs for rational anatomical lymphadenectomy. We believe that this concept makes upper mediastinal lymphadenectomy safer and more appropriate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The recurrent laryngeal nerve (RLN) lymph nodes are among the most frequently involved lymph nodes in esophageal cancer, especially in squamous cell carcinoma [1]. Surgical removal of these lymph nodes is considered beneficial for postoperative prognosis, and upper mediastinal lymph node dissection is thus a crucial part of esophagectomy for esophageal squamous cell carcinoma [2]. However, the dissection is technically demanding because the anatomical perspective in the upper mediastinum is rather complicated due to the limited space. Moreover, even microscopic injury of the RLN is associated with severe pulmonary complications and diminished quality of life with hoarseness or aspiration pneumonia postoperatively. Understanding the surgical anatomy of upper mediastinum is therefore crucial for rational lymphadenectomy that avoids RLN palsy. Unfortunately, the precise surgical anatomy of the upper mediastinum remains unclear and no distinct high-resolution images are currently available.
Recent advancements in optical technology have had a major impact on endoscopic surgery. We started performing thoracoscopic esophagectomy in the left decubitus position in 2005 [3] and then changed our approach to the prone position in 2009 [4,5,6]. Since 2015, we have been using three-dimensional (3D) high-definition endoscopy for better visualization and smoother eye-hand coordination in thoracoscopic esophagectomy. Based on the higher resolution images that this technique provides, we have developed a simple intuitive concept of upper mediastinal surgical anatomy that could facilitate rational anatomical lymphadenectomy of the RLN lymph nodes. Here, we present this concept and our surgical technique.
Concept of upper mediastinal surgical anatomy
Anatomical dissection is the division of fibrous connective fibers, the so-called “fascia,” between organs or tissues, which creates new “fasciae,” fibrous connective fibers, on both sides of the divided organs or tissues [7]. In our experience, the trachea, esophagus, left RLN (L-RLN), and right RLN (R-RLN) appear surrounded by fibrous connective tissue as a single compartment. This surrounding fibrous connective tissue is referred to as the visceral sheath [8]. Developmentally, the esophagus and trachea originate from a single embryonic structure, the anterior foregut tube, and separation from the tracheal component occurs in the human embryo between Carnegie stages 13 and 16 (28–37 days post-fertilization) [9]. The respiratory primordium and esophagus then elongate longitudinally. Therefore, the upper esophagus and trachea are seen as a single compartment.
The anatomy of the autonomic nervous system is somewhat complicated. The vagus nerve descends along the carotid artery that is outside of this visceral sheath. Then, the RLN penetrates the sheath right after diverging from the vagus nerve and subsequently runs upward along the trachea to the pharynx within the visceral sheath.
The vascular anatomy of the upper esophagus is also complicated. Many small vessels arise from the subclavian vessels and their branches such as infrathyroid vessels, internal thoracic vessels, and bronchial vessels, while the feeding arteries of the lower esophagus are predominantly the proper esophageal arteries arising from the descending aorta [10], including the right bronchial artery arising from the descending aorta as a common trunk with the third intercostal artery.
Surgical technique
All resectable esophageal cancer patients fit for esophagectomy were indicated for thoracoscopic esophagectomy. The patient is placed in a prone position under general anesthesia using a single lumen spiral endotracheal tube. First, a 12-mm camera port is inserted through the 9th intercostal space (ICS) between the scapular line and posterior axillary line using the optical access method, and the right chest cavity is inflated with a CO2 insufflation pressure of 6–8 mmHg. A 12-mm trocar is then inserted through the 7th ICS on the posterior axillary line, and two more 5-mm trocars are inserted through the 7th ICS on the scapular line and through the 5th ICS on the posterior axillary line. A 5-mm AirSeal® access port (SurgiQuest Inc., Milford, CT) is inserted through the 7th ICS on the midaxillary line, and a 12-mm trocar is inserted through the 3rd ICS on the midaxillary line. Our thoracoscopic esophagectomy with mediastinal lymphadenectomy without mini-thoracotomy is performed under 3D endoscopy using the Endoeye Flex 3D (Olympus Co., Tokyo, Japan) or the da Vinci S/Xi Surgical System (Intuitive Surgical Inc., Sunnyvale, CA). The use of daVinci Surgical System was determined if the patients agreed for private practice because there was no public insurance reimbursement during this study period. However, the procedural flow was exactly the same in both conventional thoracoscopic and robotic approaches. Our main energy device is electric cautery and vessel sealing system or ultrasonically activated device is also used where better hemostasis is required.
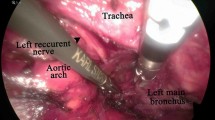
After port placement, the mediastinal pleura is incised just posterior to the esophagus at the lower mediastinum. As long as a radial surgical margin is secured, the thoracic duct is preserved, and the esophagus is mobilized from the descending aorta in the middle mediastinum. The azygos arch and right bronchial artery are routinely divided for maximum esophageal mobilization. In the upper mediastinum, careful dissection following the areolar plane between the thoracic duct and visceral sheath (Fig. 1A, B) renders the esophagus, trachea, and L-RLN lymphatic tissue a single compartment surrounded by the glittering visceral sheath (Fig. 1C). For the R-RLN nodes, division of the tracheoesophageal vessels using vessel sealing system or ultrasonically activated device from the right subclavian vessels allows for easier dissection of the loose connective tissue between the right subclavian artery and visceral sheath (Fig. 2A, B). Because the R-RLN runs just beneath the visceral sheath, incision of the visceral sheath makes isolating the R-RLN easy. After isolation of the R-RLN laterally by dividing esophageal branches of R-RLN (Fig. 2C), the surrounding lymphatic tissue is safely detached from the trachea and can be divided from the anterior tracheal lymphatic tissue. Finally, the lymphatic tissue around the R-RLN is left attached to the esophagus only (Fig. 2D).
Surgical view of the left side of the visceral sheath. A After esophageal mobilization from the thoracic duct, the tracheoesophageal vessels need to be divided. B After division of the tracheoesophageal vessels, a single compartment wrapped by the visceral sheath appears containing the L-RLN, trachea, and esophagus. C At the level of the aortic arch, the L-RLN enters the visceral sheath compartment
Surgical view around the R-RLN. A The R-RLN enters the visceral sheath compartment right after branching from the vagus nerve (R-VN). B Tracheoesophageal vessels along with pleura need to be divided. C Lateral isolation of R-RLN. The esophageal branches (arrow heads) need to be divided. D Completion of right upper mediastinal lymphadenectomy. Lymphatic tissue is attached to the lifted esophagus and the R-RLN is preserved laterally
For L-RLN node dissection, the areolar plane between the esophagus and trachea is dissected beforehand to lift the esophagus up. Then, fat tissue on the left side of the trachea (i.e., lymphatic tissue around the L-RLN) is detached from the left side of the trachea by delicate dissection of the areolar plane which is called tracheal sheath [8] (Fig. 3). Next, the esophagus is divided at the level of the aortic arch if tumor location permits. Division of the esophagus allows more mobility of the esophagus and attached lymphatic tissue toward the right side of the patient. Next, small tracheoesophageal vessels from the left subclavian vessels are divided under direct vision, and the L-RLN is isolated laterally by incising the visceral sheath (Fig. 4A) and dividing esophageal branches of L-RLN (Fig. 4B), in the same manner as for the right side. At this point, lymphatic tissue is attached to the esophagus and anterior tracheal fat tissue only. After transection of this lymphatic tissue at the same level as the esophageal transection, the very bottom of the lymphadenectomy, i.e., the anterior ends of the visceral sheath and fat tissue are divided under clear visualization of the L-RLN (Fig. 4C). The lymphatic tissue around the L-RLN is attached only to the esophagus at this point and it can be dissected up to the cervicothoracic junction. It is kept attached to esophagus and resected during cervical procedure before anastomosis (Fig. 4D).
Surgical view around the L-RLN. A Incision of fibrous connective tissue “the visceral sheath” wrapping the lymphatic tissue and L-RLN. B Division of esophageal branch (arrow heads) of L-RLN. C After esophageal transection, the proximal esophagus is lifted to expose the ventral border of the lymphadenectomy. The L-RLN has been already separated laterally. D Final view of upper mediastinal lymphadenectomy. The lymphatic tissue is attached to left side of the esophagus only. This is to be removed during cervical procedures before anastomosis
The lower part of the lymphatic tissue around the L-RLN can be easily lifted by pulling the transected anal end of the esophagus. At the subcarinal level, the visceral sheath appears to merge into the connective tissue around either the esophagus or left bronchus, and the L-RLN emerges from the sheath to join the left vagus nerve under the aortic arch. Thus, the lower part of the lymphatic tissue around the L-RLN is kept attached to the esophagus and is removed en bloc with the resected esophagus during the abdominal procedure (Video).
On completing the upper mediastinal lymphadenectomy, subcarinal and lower mediastinal lymphadenectomy is proceeded with. For reconstruction, the oral and anal ends of the esophageal stump are taped together using cotton tape before finishing the thoracoscopic procedure. The patient is then moved to the modified lithotomy position for laparoscopic creation of a gastric conduit and this is lifted via the posterior mediastinum route. Cervical anastomosis is performed either by the triangulating stapling [11] or hand-sewn technique.
Results
Between January 2009 and December 2017, thoracoscopic esophagectomy with upper mediastinal lymphadenectomy for primary esophageal cancer was performed in 189 patients: 156 men and 33 women with a median age of 66 (range 40–82) years. The clinicopathologic characteristics of the 189 patients are summarized in Table 1. Median thoracoscopic procedure time was 297 (range 205–568) min and median intraoperative blood loss was 70 ml (range unmeasurable up to 2545 ml). Median number of harvested upper mediastinal lymph nodes was 12 (cervical paraesophageal node, 101; upper thoracic paraesophageal node, 105; RLN node, 106rec; and tracheobronchial node, 106tb, according to the Japanese classification of esophageal cancer [12]). Operative results are summarized in Table 2. Postoperative complication of Clavien–Dindo classification [13] grade II or higher was observed in 86 patients (46%), while grade III or higher events were only 14%. RLN palsy of grade II or higher occurred in 20 patients (11%).
Regarding oncological outcome, 3-year overall survival rate of clinical Stage I, II, II, and IV (in union for international cancer control 8th edition) patients were 94% (n = 54), 76% (n = 58), 71% (n = 71), and 67% (n = 6), respectively, with median observation period of 1002 days (Fig. 5).
Discussion
Lymph node dissection for gastrointestinal malignancy has traditionally been performed based on vascular anatomy, with ligation of the feeding vessels at their root considered to be the geographical goal of the procedure. In 1982, Heald et al. first described the concept of total mesorectal excision (TME) for rectal cancer with improved local control [14], which focused on the fascia propria as the “holy plane” [15]. Later, Hohenberger et al. reported complete mesocolic excision (CME), defined as separation of the mesenteric plane from the parietal plane with central vascular ligation [16].
The definition of “meso” has sometimes been the subject of debate. Tufano et al. insisted that “mesorectum” was inappropriate to describe the lower part of the rectum given that “meso” was meant to refer to those peritoneal folds that, having wrapped the bowel and its vessels for a portion, were anchored to the posterior abdominal wall [17]. However, this use of “meso” in a narrow sense is applicable only to the small bowel and transverse or sigmoid colon; the posterior peritoneal fold of the ascending or descending colon is fused and atrophic, resulting in Toldt’s fascia. Nowadays, there is no argument about the existence of the mesocolon of the ascending or descending colon, and even the mesorectum is broadly accepted to refer to the fatty tissue envelop of the rectum containing blood and lymph vessels, lymph nodes, and autonomic nerves [18]. Thus, “meso” could be redefined as an anatomical unit of the gut, specifically the fatty envelope surrounded by fibrous areolar tissue or the mesothelium containing its blood vessels, lymphatic tissue, and autonomic nerve.
The term “mesoesophagus” first appeared in literature in 1998, with Matsubara et al. [19] describing it as a clearly confined compartment in the thoracocervical junction in which the RLN lymph nodes are located. They discussed the mesoesophagus mainly from the regional viewpoint and defined it as an area surrounded by the pretracheal fascia, carotid sheath, and the alar part of the prevertebral fascia, esophagus, and trachea. Based on our experience, we can safely redefine the mesoesophagus in the upper mediastinum as an anatomical unit surrounded by fibrous connective tissue containing the esophagus, trachea, tracheoesophageal vessels, lymphatic tissue, and RLN (Fig. 6). Indeed, recent histological study using cadavers demonstrated the thin membranous structure made of dense connective tissues that are considered to represent the visceral sheath in the cervicothoracic transitional region, while it was only apparent in the left side in the upper mediastinum [20]. This is consistent with the location of RLN and tracheoesophageal vessels from left and right subclavian vessels. Mesoesophagus or meso-oesophagus is also described by Cuesta et al. by reviewing surgical video and studying cadavers histologically. They showed schematic illustration of lower mediastinum [21] and this concept was followed by Akiyama et al. [22]. Others also used the term mesoesophageal resection in the context of intense dissection of periesophageal tissue without clear anatomical definition of mesoesophagus [23,24,25].
The importance of cervical paraesophageal node (101) dissection for esophageal squamous cell carcinoma is clear from Japanese nation-wide registry [2]. Therefore, it should be dissected whether three-field dissection (3FD) [26] is performed or not. Unfortunately, the definition of “field” differed among studies, and two-field dissection (2FD) in those studies often consisted of limited upper mediastinal dissection without L-RLN node (106recL) [27, 28] and usually without cervical paraesophageal nodes (101) [29,30,31]. Although there is no clear evidence available to support 3FD, recent discussion is shifted toward the role of supraclavicular node (104) dissection.
The objective of gastrointestinal cancer surgery is to remove the primary tumor with its lymphatic drainage by excising the organ-specific mesentery [32, 33], and therefore the goal of upper mediastinal lymphadenectomy should be removal of the esophageal tumor along with its mesoesophagus. However, there is considerable anatomical restriction in the mesoesophagus. The RLN and trachea must be preserved, just as the pancreas must be in gastric surgery [34,35,36]. Also, “total mesoesophageal excision” is usually not applicable because the oral end of mesoesophagus is the pharynx, which should be preserved in general. This is just similar to the case of tumor-specific mesorectal excision [37] for rectal cancer in that the gut should be preserved to some extent. As mesenteric excisions, including TME, CME, and mesogastric excision [36], are the key concept of gut surgery for malignant tumor, this mesenteric excision of esophagus would be the new principle of upper esophageal resection for esophageal cancer. The essential concept of this mesenteric excision is to mobilize mesoesophagus including RLN in an en bloc fashion and to save RLN laterally by incising visceral sheath. This is applicable identically to both right and left upper mediastinum.
RLN palsy is also an important issue in upper mediastinal lymphadenectomy. Interstudy comparison is always difficult due to the different definition and judgement of RLN palsy and the different degree of dissection. Our RLN palsy rate 11% (Clavien–Dindo Grade 2 or higher) is comparable to recent reports from Japanese groups (7–12%) [38, 39], which is acceptable considering the fact that laryngoscope was routinely performed a day after extubation in this study.
There is a limitation of this study. Because this is not a comparative study, there is no control to illustrate differences in the clinical outcomes when adopting this concept of mesenteric excision. Since surgical concept and techniques have been gradually matured and established, it is difficult to set the time point to split before and after establishment. However, our long-term outcomes are not inferior to other authorized hospitals in Japan [40].
In conclusion, we have presented our concept of mesenteric excision of upper esophagus for rational anatomical lymphadenectomy of the RLN nodes in thoracoscopic esophagectomy, which we developed based on magnified images observed during the procedure. We believe that this concept makes upper mediastinal lymphadenectomy safer and more appropriate.
Abbreviations
- RLN:
-
Recurrent laryngeal nerve
- AV:
-
Azygos vein
- TD:
-
Thoracic duct
- SCA:
-
Subclavian artery
- BA:
-
Bronchial artery
- VN:
-
Vagus nerve
- SN:
-
Sympathetic nerve
References
Udagawa H, Ueno M, Shinohara H, Haruta S, Kaida S, Nakagawa M, Tsurumaru M (2012) The importance of grouping of lymph node stations and rationale of three-field lymphoadenectomy for thoracic esophageal cancer. J Surg Oncol 106:742–747
Tachimori Y, Ozawa S, Numasaki H, Matsubara H, Shinoda M, Toh Y, Udagawa H, Fujishiro M, Oyama T, Uno T, The Registration Committee for Esophageal Cancer of the Japan Esophageal Society (2016) Efficacy of lymph node dissection by node zones according to tumor location for esophageal squamous cell carcinoma. Esophagus 13:1–7
Itami A, Watanabe G, Tanaka E, Nakayama S, Fujimoto A, Kondo M, Nakau M, Okabe H, Satoh S, Sakai Y (2008) Multimedia article. Upper mediastinal lymph node dissection for esophageal cancer through a thoracoscopic approach. Surg Endosc 22:2741
Kinjo Y, Kurita N, Nakamura F, Okabe H, Tanaka E, Kataoka Y, Itami A, Sakai Y, Fukuhara S (2012) Effectiveness of combined thoracoscopic-laparoscopic esophagectomy: comparison of postoperative complications and midterm oncological outcomes in patients with esophageal cancer. Surg Endosc 26:381–390
Tanaka E, Okabe H, Kinjo Y, Tsunoda S, Obama K, Hisamori S, Sakai Y (2015) Advantages of the prone position for minimally invasive esophagectomy in comparison to the left decubitus position: better oxygenation after minimally invasive esophagectomy. Surg Today 45:819–825
Tanaka E, Okabe H, Tsunoda S, Obama K, Kan T, Kadokawa Y, Akagami M, Sakai Y (2012) Feasibility of thoracoscopic esophagectomy after neoadjuvant chemotherapy. Asian J Endosc Surg 5:111–117
Sakai Y, Hasegawa S, Shinohara H, Yamada M, Michiaki O (2016) Laparoscopic total mesorectal excision (TME) for rectal cancer. In: Sakai Y (ed) Laparoscopic surgery for colorectal cancer. Springer, Tokyo, pp 109–135
Sarrazin R, Voog R (1971) Anatomical background to mediastinoscopy. In: Jepsen O, Sørensen HR (eds) Mediastinoscopy. Odense University Press, Odense, pp 1–6
Sadler TW (2014) Langman’s medical embryology, 13th edn. LWW, Philadelphia
Salassa JR, Pearson BW, Payne WS (1977) Gross and microscopical blood supply of the trachea. Ann Thorac Surg 24:100–107
Noshiro H, Urata M, Ikeda O, Iwasaki H, Nabae T, Uchiyama A, Nagai E, Tanaka M (2013) Triangulating stapling technique for esophagogastrostomy after minimally invasive esophagectomy. Surgery 154:604–610
Japan Esophageal Society (2017) Japanese Classification of Esophageal Cancer, 11th edition: part I. Esophagus 14:1–36
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Heald RJ, Husband EM, Ryall RD (1982) The mesorectum in rectal cancer surgery—the clue to pelvic recurrence? Br J Surg 69:613–616
Heald RJ (1988) The ‘Holy Plane’ of rectal surgery. J R Soc Med 81:503–508
Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S (2009) Standardized surgery for colonic cancer: complete mesocolic excision and central ligation—technical notes and outcome. Colorectal Dis 11:354–364 (discussion 364–355)
Tufano A, Tufano G, Brusciano L, del Genio G, Rossetti G, Di Stazio C, Grillo M, del Genio A (2007) Mesorectum, is it an appropriate term? Int J Colorectal Dis 22:1127–1128
Havenga K, Grossmann I, DeRuiter M, Wiggers T (2007) Definition of total mesorectal excision, including the perineal phase: technical considerations. Dig Dis 25:44–50
Matsubara T, Ueda M, Nagao N, Takahashi T, Nakajima T, Nishi M (1998) Cervicothoracic approach for total mesoesophageal dissection in cancer of the thoracic esophagus. J Am Coll Surg 187:238–245
Tokairin Y, Nakajima Y, Kawada K, Hoshino A, Okada T, Ryotokuji T, Okuda M, Kume Y, Kawamura Y, Yamaguchi K, Nagai K, Akita K, Kinugasa Y (2018) Histological study of the thin membranous structure made of dense connective tissue around the esophagus in the upper mediastinum. Esophagus 15:272–280
Cuesta MA, Weijs TJ, Bleys RL, van Hillegersberg R, van Berge Henegouwen MI, Gisbertz SS, Ruurda JP, Straatman J, Osugi H, van der Peet DL (2015) A new concept of the anatomy of the thoracic oesophagus: the meso-oesophagus. Observational study during thoracoscopic esophagectomy. Surg Endosc 29:2576–2582
Akiyama Y, Iwaya T, Endo F, Nikai H, Sato K, Baba S, Chiba T, Kimura T, Takahara T, Otsuka K, Nitta H, Mizuno M, Kimura Y, Koeda K, Sasaki A (2018) Thoracoscopic esophagectomy with total meso-esophageal excision reduces regional lymph node recurrence. Langenbecks Arch Surg. https://doi.org/10.1007/s00423-018-1727-5
Tachimori Y (2014) Total mesoesophageal esophagectomy. Chin Med J (Engl) 127:574–579
Lin J, Kang M, Chen S, Deng F, Han Z (2016) Feasibility and strategy for left tracheobronchial lymph node dissection in thoracolaparoscopic esophageal cancer surgery. Thorac Cancer 7:199–206
Kang M, Huang S, Lin J, Chen S, Han W (2016) Video-assisted thoracoscopy the total mesoesophageal excision and systematic en bloc mediastinal lymph node dissection. J Vis Surg 2:102
Akiyama H, Tsurumaru M, Udagawa H, Kajiyama Y (1994) Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg 220:364–372 (discussion 372–363)
Nishihira T, Hirayama K, Mori S (1998) A prospective randomized trial of extended cervical and superior mediastinal lymphadenectomy for carcinoma of the thoracic esophagus. Am J Surg 175:47–51
Fujita H, Sueyoshi S, Tanaka T, Fujii T, Toh U, Mine T, Sasahara H, Sudo T, Matono S, Yamana H, Shirouzu K (2003) Optimal lymphadenectomy for squamous cell carcinoma in the thoracic esophagus: comparing the short- and long-term outcome among the four types of lymphadenectomy. World J Surg 27:571–579
Igaki H, Tachimori Y, Kato H (2004) Improved survival for patients with upper and/or middle mediastinal lymph node metastasis of squamous cell carcinoma of the lower thoracic esophagus treated with 3-field dissection. Ann Surg 239:483–490
Kato H, Watanabe H, Tachimori Y, Iizuka T (1991) Evaluation of neck lymph node dissection for thoracic esophageal carcinoma. Ann Thorac Surg 51:931–935
Fang WT, Chen WH, Chen Y, Jiang Y (2007) Selective three-field lymphadenectomy for thoracic esophageal squamous carcinoma. Dis Esophagus 20:206–211
Gilchrist RK, David VC (1938) Lymphatic spread of carcinoma of the rectum. Ann Surg 108:621–642
Coller FA, Kay EB, Macintyre RS (1941) Regional lymphatic metastases of carcinoma of the colon. Ann Surg 114:56–67
Shinohara H, Kurahashi Y, Kanaya S, Haruta S, Ueno M, Udagawa H, Sakai Y (2013) Topographic anatomy and laparoscopic technique for dissection of no. 6 infrapyloric lymph nodes in gastric cancer surgery. Gastric Cancer 16:615–620
Shinohara H, Haruta S, Ohkura Y, Udagawa H, Sakai Y (2015) Tracing dissectable layers of mesenteries overcomes embryologic restrictions when performing infrapyloric lymphadenectomy in laparoscopic gastric cancer surgery. J Am Coll Surg 220:e81–e87
Shinohara H, Kurahashi Y, Haruta S, Ishida Y, Sasako M (2018) Universalization of the operative strategy by systematic mesogastric excision for stomach cancer with that for total mesorectal excision and complete mesocolic excision colorectal counterparts. Ann Gastroenterol Surg 2:28–36
Zaheer S, Pemberton JH, Farouk R, Dozois RR, Wolff BG, Ilstrup D (1998) Surgical treatment of adenocarcinoma of the rectum. Ann Surg 227:800–811
Oshikiri T, Nakamura T, Hasegawa H, Yamamoto M, Kanaji S, Yamashita K, Matsuda T, Sumi Y, Fujino Y, Tominaga M, Suzuki S, Kakeji Y (2018) Standardizing procedures improves and homogenizes short-term outcomes after minimally invasive esophagectomy. Langenbecks Arch Surg 403:221–234
Saito Y, Takeuchi H, Fukuda K, Suda K, Nakamura R, Wada N, Kawakubo H, Kitagawa Y (2018) Size of recurrent laryngeal nerve as a new risk factor for postoperative vocal cord paralysis. Dis Esophagus https://doi.org/10.1093/dote/dox162
Motoyama S, Maeda E, Yano M, Yasuda T, Ohira M, Doki Y, Toh Y, Higashi T, Matsubara H, Society JE (2018) Appropriateness of the institute certification system for esophageal surgeries by the Japan Esophageal Society: evaluation of survival outcomes using data from the National Database of Hospital-Based Cancer Registries in Japan. Esophagus. https://doi.org/10.1007/s10388-018-0646-4
Acknowledgements
This work was supported in part by JSPS KAKENHI Grant Number 16H05399.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Shigeru Tsunoda, Hisashi Shinohara, Seiichiro Kanaya, Hiroshi Okabe, Eiji Tanaka, Kazutaka Obama, Hisahiro Hosogi, Shigeo Hisamori, and Yoshiharu Sakai have no conflict of interest or financial ties to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tsunoda, S., Shinohara, H., Kanaya, S. et al. Mesenteric excision of upper esophagus: a concept for rational anatomical lymphadenectomy of the recurrent laryngeal nodes in thoracoscopic esophagectomy. Surg Endosc 34, 133–141 (2020). https://doi.org/10.1007/s00464-019-06741-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-06741-x