Abstract
Background
The aim of the present study was to explore incidence and severity of parastomal hernia (PSH) formation during the first 2 years after open/laparoscopic abdominoperineal resection (APR).
Methods
This was a retrospective cohort study conducted in a single institution. All patients who underwent laparoscopic/open APR for low rectal cancer within a 10-year study period were assessed for study eligibility.
Results
In total, 148 patients were included in the study (97 patients after laparoscopic APR; 51 patients after open APR). There were no statistically significant differences between study subgroups regarding demographic and clinical features. The incidence of PSH detected by physical examination was significantly higher in patients after laparoscopic APR 1 year after the surgery (50.5% vs. 19.6%, p < 0.001) and 2 years after the surgery (57.7% vs. 29.4%, p = 0.001). The incidence of radiologically detected PSH was significantly higher in laparoscopically operated patients after 1 year (58.7% vs. 35.3%, p = 0.007) and after 2 years (61.8% vs. 37.2%, p = 0.004). The mean diameter of PSH was similar in both study subgroups. The incidence of incisional hernia was significantly higher in patients who underwent open APR after 1 year (25.5% vs. 7.2%, p = 0.002) and after 2 years (31.3% vs. 7.2%, p < 0.001).
Conclusions
The risk of PSH development after laparoscopic APR appears to be significantly higher in comparison with patients undergoing open APR. Higher incidence of PSH should be considered a potential disadvantage of minimally invasive approach to patients with low rectal cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Parastomal hernia (PSH) is a frequent complication following the formation of an ostomy, which typically occurs within 2 years after stoma creation [1, 2]. PSH presents a complex surgical problem because of its high occurrence, technical difficulty to repair and high recurrence rates after surgical repair [3]. End colostomies have the highest incidence of all hernia formation, ranging from 4 to 56% depending on the diagnostic criteria, definition of hernia etc. [4,5,6]. PSH aggravates the inherently diminished quality of life of stoma patients due to abdominal pain, discomfort, poor fitting of the pouching system with leakage of intestinal contents, and skin excoriation [7,8,9,10].
There are several risk factors which have been reported to increase the incidence of PSH—age, obesity, malnutrition, increased intra-abdominal pressure, postoperative sepsis, emergency surgery, steroids, and chronic obstructive pulmonary disease [5, 11]. Minimally invasive surgery presents a safe and feasible approach to low rectal cancer, which is associated with decreased postoperative pain and morbidity, more rapid recovery, and better quality of life within the first few months after surgery [12,13,14,15,16]. However, it has been suggested that laparoscopic creation of colostomy might be associated with higher incidence of PSH formation [17, 18].
To the best of our knowledge, there has been no study in the available literature focused on the investigation of PSH incidence following laparoscopic vs. open abdominoperineal resection (APR). Moreover, there are only few studies available investigating PSH incidence at the site of terminal colostomy as a primary outcome measure, which may have caused an underpowered analysis of published studies.
The aim of the present study was to explore incidence and severity of PSH formation during the first 2 years after open/laparoscopic APR.
Materials and methods
Design and setting
This was a retrospective cohort study conducted in the University Hospital Ostrava, Czech Republic. The study was approved by the Ethics Committee of the University Hospital Ostrava. All patients who underwent laparoscopic/open APR with total mesorectal excision (TME) for low rectal cancer within a 10-year study period (1 January 2006–31 December 2015) were assessed for study eligibility. Exclusion criteria were patient’s death or incomplete data regarding 2-year postoperative follow-up period. Management of all patients was based on multidisciplinary team meetings and practiced in accordance with NCCN guidelines.
Surgical technique
Surgery was performed after standard preoperative bowel cleansing and antibiotic prophylaxis. The location of the colostomy was marked preoperatively by an experienced stoma nurse in all cases. Patients were placed in Lloyd-Davies position; trocars were placed in the standardized manner. After abdominal cavity exploration, the inferior mesenteric vessels were identified and divided. Mobilization of the left colon and splenic flexure was done. TME was performed according to the principles stated by Heald [19]. The sigmoid colon was divided (at the site of the future colostomy) using an endostapler; the specimen was extracted through the perineal wound. The terminal colostomy was constructed through the rectus abdominis muscle at the preoperatively marked skin site (by and experienced wound ostomy nurse). There were no differences in colostomy construction between study subgroups (laparoscopic vs. open ARP). Standard peri-operative care was offered to all patients.
Data collection
The demographic data and clinical characteristics of all study patients (age, sex, body mass index, ASA classification and cancer stage), type of surgery, and post-operative 30-day morbidity were extracted from medical records.
PSH presence/absence was assessed by means of clinical and radiological examination at two time points: (i) 1 year after APR and (ii) 2 years after APR. Clinical examination of all study patients was performed during an outpatient coloproctology clinic attendance by an experienced colorectal surgeon (all patients were examined in the standing and decubitus positions). PSH detected by physical examination was defined as clinical PSH. Clinical examination was also aimed to identify potential incisional hernia in study patients (incisional hernia in midline laparotomy after open APR or port-site incisional hernia after laparoscopic APR).
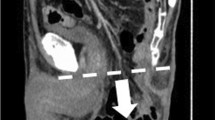
Radiological examination was performed by means of abdominal computed tomography (CT); intravenous contrast was employed in most cases. Each CT was examined by a single experienced radiologist looking exclusively for peristomal pathology. The radiologist was blind to the results of the clinical examination. Size (diameter) of PSH on CT scans was recorded. According to the classification system proposed by Moreno-Matias et al. in 2009 [20], three types of PSH were distinguished (based on the assessment of the content and size of hernia sac): Type I (hernia sac contains only the bowel forming the stoma), Type II (hernia sac contains omentum), and Type III (hernia sac contains an intestinal loop other than that forming the stoma).
Statistical analysis
The acquired data underwent analysis by means of descriptive statistics. The differences between the subgroups were tested using the Chi square test and Fisher’s exact test for categorical variables. For continuous variables, test of normality was performed and the two-sample t test was used to test the differences and non-parametric two-sample Mann–Whitney test. A level of significance of α = 0.05 and p values < 0.05 were considered statistically significant. Statistical analysis was performed by Stata version 13.
Results
Within a study period (1 January 2006–31 December 2015), 172 patients underwent laparoscopic/open APR for rectal cancer and met the inclusion criteria. Of these, 24 patients (13.9%) were excluded due to the study design and exclusion criteria. There were 17 conversions (9.8%) from laparoscopic to open APR; these patients were analyzed within the open APR study subgroup. The most frequent reason for conversion was non-progressive surgery due to inadequate operative visual field (narrow pelvis, huge tumor).
In total, 148 patients were included in the study and underwent analysis. There were 97 patients after laparoscopic APR and 51 patients after APR performed through laparotomy. The basic demographic and clinical features are presented in Table 1; there were no statistically significant differences between both study groups regarding age, gender, BMI, ASA grade, cancer stage, operative time, and 30-day postoperative morbidity.
Clinical PSH (parastomal hernia revealed by physical examination) was detected in 59 study patients (39.8%) 1 year after APR, and in 71 patients (47.8%) 2 years after APR (Table 2). The incidence of clinical PSH was significantly higher in patients who underwent laparoscopic APR in comparison with open APR at both time points—1 year after the surgery (50.5% vs. 19.6%, p < 0.001) and 2 years after the surgery (57.7% vs. 29.4%, p = 0.001).
The incisional hernia (hernia in midline laparotomy after open APR or port-site incisional hernia after laparoscopic APR) was found in 20 study patients (13.5%) after 1 year and in 23 patients (15.5%) after 2 years (Table 2). The incidence of incisional hernia was significantly higher in patients who underwent open APR 1 year after the surgery (25.5% vs. 7.2%, p = 0.002); the same applies to the difference between study subgroups after 2 years (31.3% vs. 7.2%, p < 0.001).
Radiologically detected PSH was found in 75 study patients (50.6%) 1 year after APR, and in 79 patients (53.3%) 2 years after APR. Data regarding particular types of PSH according to Moreno-Matias classification detected in our study subgroups are clearly presented in Table 3. The incidence of radiologically detected PSH was significantly higher in the subgroup of patients who underwent laparoscopic APR—1 year after the surgery (58.7% vs. 35.3%, p = 0.007) and 2 years after the surgery (61.8% vs. 37.2%, p = 0.004). The differences between both study subgroups were conditioned mainly by a higher incidence of PSH type II and III.
The mean diameter of radiologically detected PSH was 8.9 ± 0.44 cm 1 year after APR and 11.3 ± 0.49 cm 2 years after APR (Table 3). The mean diameter of PSH was higher in a subgroup of laparoscopically operated patients in both study periods, but the differences were not statistically significant (p = 0.223 and p = 0.305).
Discussion
PSH presents the most common complication of stoma formation, which adversely affects a patient’s quality of life, psychological well-being and healthcare resources [1, 4, 21, 22]. According to literature, a wide range of PSH rates is reported as a result of varying definitions, methods of diagnosis, length of follow-up, and type of stoma [1, 4,5,6,7, 23,24,25]. A meta-analysis has estimated the PSH incidence to be in the region of 30% for end ileostomy and approximately 50% for end colostomy at 10 years [4].
In our study group, both methods of PSH assessment (clinical and radiological examination) revealed a high frequency of PSH. One year after the surgery, PSH was detected by clinical examination in 39.8% of patients, and in 50.6% of patients by radiological examination. After 2 years, there were 47.8% of patients with clinical PSH and 53.3% of patients with radiological PSH. We suppose that the rather high PSH incidence in our study group could be attributed to our study design—PSH incidence/severity investigation was a primary outcome measure of the study.
Although some patients with PSH are asymptomatic, roughly three-quarters of patients with PSH exhibit clinical symptoms related to their hernia [7, 9]. Moreover, unrepaired PSH can lead to bowel obstruction, incarceration, or possible strangulation [7,8,9,10, 21, 23, 24]. Symptomatic PSH is an indication for surgery. According to available data coming from systematic reviews, mesh repair is the golden standard currently [21, 26,27,28]. However, there is a lack of evidence with respect to the ideal technique of stoma formation or the ideal technique of PSH mesh repair. Multi-institutional randomized controlled trials comparing the safety and efficacy of different surgical techniques are, therefore, essential in order to draw definitive conclusions [5, 21, 24].
A diagnosis of PSH is made either through clinical examination or via imaging modalities. Although there is no golden standard for PSH diagnosis, an abdominal CT has been the traditional imaging modality to confirm the diagnosis or to obtain a better characterization of the PSH [20, 24, 29]. In our study, the analysis of diagnostic methods used for PSH detection revealed a high correlation between clinical and radiological PSH incidence (39.8% vs. 50.6% after 1 year; 47.8% vs. 53.3% after 2 years). In accordance with the available literature, the use of abdominal CT can detect smaller PSH that are not apparent during clinical examination [20, 29]. Moreno-Matias et al. in their study concluded that clinico-radiological concordance is high for PSH types II and III, indicating that radiology gives objectivity to the clinical findings. The correlation for PSH types Ia and Ib is lower, but these PSH are usually not associated with symptoms, since the hernial sac contains only the bowel loop forming the stoma [20].
Our data revealed a significantly higher incidence of PSH in patients who underwent laparoscopic APR, which presents the most important outcome of the study. We have detected an approximately twofold higher incidence of clinical PSH (50.5% vs. 19.6% after 1 year; 57.7% vs. 29.4% after 2 years) and radiologically detected PSH (58.7% vs. 35.3% after 1 year; 61.8% vs. 37.2% after 2 years) in patients undergoing laparoscopic APR. The first time that laparoscopic surgery was linked to a higher incidence of PSH was within a review paper by Carne et al. in 2003 [18]. Authors reported the outcomes of a literature search focused on data regarding PSH following minimally invasive stoma formation. Carne et al. concluded that the incidence of PSH after laparoscopic stoma formation remains unclear and further studies are required.
In the available literature, there is only one study focused on the comparison of PSH incidence after laparoscopic vs. open colorectal surgery [17]. Randall et al. detected PSH in 18% of patients after laparoscopic approach compared to 2% of patients after open procedures. However, there are several concerns regarding the design of the study published by Randall et al.—the study population was extremely heterogeneous (emergency and elective procedures, patients with cancer and benign diseases, patients with ileostomy and colostomy) and a limited number of patients were included (33 patients after laparoscopic vs. 44 patients after open surgery).
In our study, patients who underwent laparoscopic APR had a significantly higher incidence of PSH and a significantly lower incidence of incisional hernia. We suppose that these outcomes are the consequence of predominant hernia formation in the weakest point of the abdominal wall. The defect created for terminal colostomy presents the weakest point of abdominal wall in laparoscopically operated patients, owing to this, PSH develops predominantly in patients after laparoscopic APR. On the other hand, laparotomy presents the weakest point of abdominal wall in patients after open APR (newly-emerged incisional hernia in these patients is associated with the release of increased intra-abdominal pressure; therefore, PSH does not arise subsequently).
The high incidence of PSH together with the unsatisfactory outcomes of its repair (morbidity associated with any corrective operation) has led to a novel idea with emphasis on prevention—the use of peristomal prophylactic mesh at the time of the initial operation [21, 30, 31]. According to recent meta-analysis, reinforcement of a stoma with a biological or synthetic mesh at the time of its formation significantly reduces the PSH incidence with no increase in morbidity [32]. Authors conclude that reinforcing elective stomas with mesh reduces subsequently PSH rates, complications, repairs and saves money. We suppose that a two-fold higher PSH incidence after laparoscopic APR (in comparison with open APR) could be a strong argument for the application of peristomal prophylactic mesh during laparoscopic APR in an effort to reduce the rates of PSH formation.
The present study was focused on the investigation of PSH incidence/severity within the first 2 years after open/laparoscopic APR. A retrospective cohort study design presents the main limitation of the study. However, the proposed study was sufficiently powered, a high homogeneity of study group was achieved, two methods of PSH examination (clinical and radiological) at fixed time-points were employed, and a standardized classification system for PSH assessment was used.
In conclusion, the risk of PSH development after laparoscopic APR appears to be significantly higher in comparison with patients undergoing open APR. Although many stoma-related complications are not increased by the use of laparoscopic surgery, higher incidence of PSH should be considered a potential disadvantage of minimally invasive approach to patients with low rectal cancer. A promising method of reducing the incidence and consequences of this significant complication is the use of prophylactic peristomal meshes at the time of the initial laparoscopic operation.
References
Londono-Schimmer EE, Leaong AP, Phillips RK (1994) Life table analysis of stomal complications following colostomy. Dis Colon Rectum 37(9):916–920
Gillern S, Bleier JI (2014) Parastomal hernia repair and reinforcement: the role of biologic and synthetic materials. Clin Colon Rectal Surg 27:162–171
Hansson BME, Morales-Conde S, Mussack T, Valdes J, Muysoms FE, Bleichrodt RP (2013) The laparoscopic modified Sugarbaker technique is safe and has a low recurrence rate: a multicenter cohort study. Surg Endosc 27:494–500
Carne PW, Robertson GM, Frizelle FA (2003) Parastomal hernia. Br J Surg 90(7):784–793
O’Neill CH, Borrazzo EC, Hyman NH (2015) Parastomal hernia repair. J Gastrointest Surg 19:766–769
Cheung MT, Chia NH, Chiu WY (2001) Surgical treatment of parastomal hernia complicating sigmoid colostomies. Dis Colon Rectum 44:266–271
Ripoche J, Basurko C, Fabbro-Perray P, Prudhomme M (2011) Parastomal hernia. A study of the French federation of ostomy patients. J Visc Surg 148:e435–e441
Asif A, Ruiz M. Yetasook A, Denham W, Linn J, Carbray J, Ujiki MB (2012) Laparoscopic modified Sugerbaker technique results in superior recurrence rate. Surg Endosc 26:3430–3434
Kald A, Juul KN, Hjortsvang H, Sjodahl RI (2008) Quality of life is impaired in patients with peristomal bulging of a sigmoid colostomy. Scand J Gastroenterol 43:627–633
Ihnát P, Guňková P, Peteja M, Vávra P, Pelikán A, Zonča P (2016) Diverting ileostomy in laparoscopic rectal cancer surgery: high price of protection. Surg Endosc 30(11):4809–4816
Funahashi K, Suzuki R, Nagashima Y, Matsuda S, Koike J, Shiokawa H, Ushigome M, Arai K, Kaneko T, Kurihara A, Kaneko H (2014) Risk factors for parastomal hernia in Japanese patients with permanent colostomy. Surg Today 44:1465–1469
Vennix S, Pelzers L, Bouvy N, Beets GL, Pierie JP, Wiggers T, Breukink S (2014) Laparoscopic versus open total mesorectal excision for rectal cancer. Cochrane Database Syst Rev 4:CD005200
Tan WJ, Chew MH, Dharmawan AR, Singh M, Acharyya S, Loi CT, Tanq CL (2016) Critical appraisal of laparoscopic vs open rectal cancer surgery. World J Gastrointest Surg 8(6):452
Ng SS, Lee JF, Yiu RY, Li JC, Hon SS, Mak TW, Leung WW, Leunq KL (2014) Long-term oncologic outcomes of laparoscopic versus open surgery for rectal cancer: a pooled analysis of 3 randomized controlled trials. Ann Surg 259(1):139–147
Agha A, Benseler V, Hornung M, Gerken M, Iesalnieks I, Fürst A, Anthuber M, Jauch KW, Schlitt HJ (2014) Long-term oncologic outcome after laparoscopic surgery for rectal cancer. Surg Endosc 28(4):1119–1125
Ihnát P, Martínek L, Mitták M, Vávra P, Ihnát Rudinská L, Zonča P (2014) Quality of life after laparoscopic and open resection of colorectal cancer. Dig Surg 31(3):161–168
Randall J, Lord B, Fulham J, Soin B (2012) Parastomal hernias as the predominant stoma complication after laparoscopic colorectal surgery. Surg Laparosc Endosc Percutan Tech 22(5):420–423
Carne PW, Frye JN, Robertson GM, Frizelle FA (2003) Parastomal hernia following minimally invasive stoma formation. ANZ J Surg 73(10):843–845
Heald RJ, Husband EM, Ryall RD (1982) The mesorectum in rectal cancer surgery—the clue to pelvic recurrence? Br J Surg 69:613–616
Moreno-Matias J, Serra-Aracil X, Darnell-Martin A, Bombardo-Junca J, Mora-Lopez L, Alcantara-Moral M, Rebasa P, Ayguavives-Garnica I, Navarro-Soto S (2009) The prevalence of parastomal hernia after formation of an end colostomy. A new clinico-radiological classification. Colorectal Dis 11:173–177
Hotouras A, Murphy J, Thaha M, Chan CL (2013) The persistent challenge of parastomal herniation: a review of the literature and future developments. Colorectal Dis 15(5):e202–e214
Malik T, Lee MJ, Harikrishnan AB (2018) The incidence of soma related morbidity—a systematic review of randomised controlled trials. Ann R Coll Surg Engl 100(7):501–508. https://doi.org/10.1308/rcsann.2018.0126 [Epub ahead of print]
Petersson J, Koedam TW, Bonjer HJ, Andersson J, Angenete E, Bock D, Cuesta MA, Deijen CL, Furst A, Lacz AM, Rosenberg J, Haglind E, COlorectal cancer Laparoscopic or Open Resection (COLOR) II Study Group (2018) Bowel obstruction and ventral hernia after laparoscopic versus open surgery for rectal cancer in a randomized trial (COLOR II). Ann Surg. https://doi.org/10.1097/SLA.0000000000002790 [Epub ahead of print]
Aguina CT, Iannuzzi JC, Probst CP, Kelly KN, Noyes K, Fleming FJ, Monson JRT (2014) Parastomal hernia: a growing problem with new solutions. Dig Surg 31:366–376
Ihnát P, Ihnát Rudinská L, Zonča P (2014) Radiofrequency energy in surgery: state of the art. Surg Today 44(6):985–991
Hansson BM, Slater NJ, van der Velden AS, Groenewoud HM, Buyne OR, de Hingh IH, Bleichrodt RP (2012) Surgical techniques for parastomal hernia repair: a systematic review of the literature. Ann Surg 255:685–695
Slater NJ, hansson BME, Buyne OR, Hendriks T, Bleichrodt RP (2011) Repair of parastomal hernias with biological grafts: a systematic review of the literature. J Gastrointest Surg 15:1252–1258
Hansson BME (2013) Parastomal hernia: treatment and prevention 2013; where do we go from here? Colorectal Dis 15:1467–1470
Cingi A, Carik T, Sever A, Aktan AO (2006) Enterostomy site hernias: a clinical and computerized tomographic evaluation. Dis Colon Rectum 49:1559–1563
Janson AR, Janes A, Israelsson LA (2010) Laparoscopic stoma formation with a prophylactic prosthetic mesh. Hernia 14:495–498
Jones HG, Rees M, Aboumarzouk OM, Brown J, Cragg J, Billings P, Carter B, Chandran P (2018) Prosthetic mesh placement for the prevention of parastomal herniation. Cochrane Database Syst Rev 7:CD008905
Findlay JM, Wood CPJ, Cunninham C (2018) Prophylactic mesh reinforcement of stomas: a cost-effectiveness meta-analysis of randomised controlled trials. Tech Coloproctol. https://doi.org/10.1007/s10151-018-1774-5 [Epub ahead of print]
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Peter Ihnát, Lubomír Tulinský, Tomáš Jonszta, Pavel Koscielnik, Lucia Ihnát Rudinská, and Igor Penka have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Ihnát, P., Tulinský, L., Jonszta, T. et al. Parastomal and incisional hernia following laparoscopic/open abdominoperineal resection: is there a real difference?. Surg Endosc 33, 1789–1794 (2019). https://doi.org/10.1007/s00464-018-6453-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-6453-0