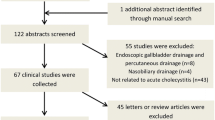
Abstract
Background
Percutaneous cholecystostomy tube (PTGBD), endoscopic retrograde cholangiopancreatography with transpapillary gallbladder drainage (TP), and endoscopic ultrasound-guided transmural gallbladder drainage (EGBD) using lumen-apposing metal stents (LAMS) have been offered for gallbladder decompression for acute cholecystitis in high-risk surgical patients. Yet, there are limited data comparing these therapies. Our aim was to compare the safety and efficacy of EGBD to TP and PTGBD for gallbladder drainage.
Methods
We retrospectively collected high-risk surgical patients from six centers with acute cholecystitis who underwent gallbladder drainage by EGBD, TP, or PTGBD. Data included technical success (gallbladder drainage), clinical success (acute cholecystitis resolution), adverse events (AE), and follow-up.
Results
From 2010 to 2016, 372 patients underwent gallbladder drainage, with 146 by PTGBD, 124 by TP, and 102 drained by EGBD. Technical (98% vs. 88% vs. 94%; p = 0.004) and Clinical (97% vs. 90% vs. 80%; p < 0.001) success rates were significantly higher with PTGBD and EGBD compared to TP. PTGBD group had statistically significantly higher number of complications as compared to EGBD and TP groups (2 0% vs. 2% vs. 5%; p = 0.01). Mean hospital stay in the EGBD group was significantly less than TP and PTGBD (16 vs. 18 vs. 19 days; p = 0.01), while additional surgical intervention was significantly higher in the PTGBD group compared to the EGBD and TP groups (49% vs. 4% vs. 11%; p < 0.0001).
Conclusions
EGBD with LAMS is an effective and safer alternative to TP and PTGBD for treatment of patients with acute cholecystitis who cannot undergo surgery. EGBD with LAMS has significantly lower overall AEs, hospital stay, and unplanned admissions compared to PTGBD. Trial registration: ClinicalTrials.gov Identifier: NCT01522573.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Laparoscopic or conventional cholecystectomy is the standard treatment for acute calculous or acalculous cholecystitis, with an operative mortality rate of < 0.8% [1]. However, in patients with significant comorbidities, emergent cholecystectomy can result in morbidity up to 41% and perioperative mortality up to 18% [2]. In such patients, non-surgical gallbladder drainage options including percutaneous gallbladder drainage (PTGBD) via cholecystostomy tube placement and endoscopic transpapillary drainage by gallbladder stenting (TP) are safe and effective procedures [3,4,5]. Not only can these procedures be effective for acute cholecystitis, but also are a feasible strategy for long-term management of symptomatic cholelithiasis in patients who are poor surgical candidates [6].
Percutaneous transhepatic gallbladder drainage (PTGBD) has a high clinical success rate for the temporary decompression of the gallbladder (57–100%) [7]. However, this procedure may not be possible in patients with severe coagulopathy, thrombocytopenia, or anatomically inaccessible gallbladders. Additional risks include catheter dislodgment, cellulitis, bleeding, fistulas, and infection [8,9,10]. The external catheter may also lead to significant pain, adversely affecting the patient’s quality of life. PTGBD also needs to be performed repeatedly, as the percutaneous drain may need to be upsized. Moreover, there is a greater than 50% recurrence rate of cholecystitis when the catheter is removed [8, 10, 11].
Transpapillary drainage by ERCP (TP) accesses the gallbladder lumen through the cystic duct with a wire and placement of a transpapillary, transcystic double-pigtail 7F–10F plastic stent. The technical success rates of this method vary, a systematic review revealed pooled success of 81–96% [5, 12,13,14,15]. However, this technique may not be possible if the cystic duct cannot be opacified during a cholangiogram or the guidewire cannot be advanced through the cystic duct into the gallbladder due to tortuosity or obstruction [16, 17].
Endoscopic ultrasound-guided gallbladder drainage (EUS-GBD) has been proposed as an alternative drainage procedure to PTGBD and TP. EUS-GBD is highly effective with technical and clinical success rates of > 95% and 93%, respectively. Recently, the use of the novel lumen-apposing fully covered self-expandable metal stents (LAMS) has been used to perform EUS-GBD by creating a newly formed fistula track by the stent expansion. LAMS are able to create a stable anastomosis between the gallbladder lumen and the gut lumen, thus allowing for effective enteric drainage of the gallbladder [18, 19].
It is currently unclear how EUS-GBD using LAMS compares with percutaneous cholecystostomy and transpapillary drainage by ERCP for safe and effective drainage of the gallbladder in high-risk patients. The aim of the current study was to compare clinical success, technical success, and adverse events of EGBD with percutaneous cholecystostomy and endoscopic transpapillary drainage as a management approach for acute cholecystitis in patients who were unfit for surgery.
Methods
Study aims
The primary aim was to compare the clinical success and safety of the three treatment groups. Clinical success was defined by improvement in the patient’s overall clinical picture within 5 days of the procedure based on the patient being afebrile, resolution of leukocytosis (WBC < 11.0 × 109/L), resolution of abdominal pain, and ability to tolerate oral intake within 5 days of the procedure.
Procedural adverse events were defined as those that occurred within 7 days of procedure. Procedure-related death was defined as death resulting from events directly related to the procedure. Long-term adverse events were defined as only biliary tract-related adverse events that occurred after the 7-day period of the initial procedure. The severity of adverse events was graded according to the ASGE lexicon of endoscopic adverse events [20].
Patients
Data were collected retrospectively in a study of consecutive patients in six tertiary care centers (five in the United States and one International) between May 2010 and June 2016 with acute cholecystitis, in patients, not fit for a cholecystectomy based in the majority of cases upon the ACS NSQIP Surgical Risk Calculator in conjunction with the surgeons’ clinical judgement. The diagnosis of acute cholecystitis was based on the Tokyo Guidelines [21]. Patients were initially managed with bowel rest, intravenous fluids, and antibiotics without improvement. Since all patients were deemed to be non-operative candidates based upon their comorbidities, gallbladder drainage was performed by one of the three minimally invasive procedures: percutaneous gallbladder drainage (PGBD), endoscopic retrograde cholangiopancreatography with transpapillary drainage (TP), or endoscopic ultrasound-guided transmural gallbladder drainage using lumen-apposing metal stents (EGBD) [21, 22]. The choice of gallbladder drainage was based upon the discretion of the primary physician (in most cases the surgeon) after careful consultation with the staff gastroenterologist and interventional radiologist. The study was approved by the Institutional Review Board of all the institutions.
Procedure technique
Percutaneous cholecystostomy tube
All procedures were performed under local anesthesia. The percutaneous cholecystostomy was performed under ultrasound and fluoroscopic guidance via a transhepatic or transperitoneal approach. An 18-gage needle was used to puncture the gallbladder lumen under direct ultrasound guidance and bile was aspirated to confirm position. Contrast medium was then used to opacity the gallbladder lumen, a 0.035″ guidewire was passed through the needle and coiled inside the gallbladder lumen. The tract was dilated and a pigtail drainage catheter (8- to 10-Fr) was placed into the gallbladder lumen over the guidewire.
Endoscopic transpapillary gallbladder drainage
To perform transpapillary gallbladder drainage, a duodenoscope was used and biliary cannulation was done. Endoscopic sphincterotomy was done in all patients. Both a biliary sphincterotomy and a balloon occlusion cholangiography were used to identify the origin of the cystic duct take off. A guidewire (Dreamwire; Boston Scientific, or Glidewire; Terumo, Somerset, NJ, or Visiglide; Olympus) was advanced into the cystic duct using various catheters including a standard sphincterotome, an extraction balloon, or a swing tip catheter (Olympus America) to obtain access to the gallbladder. Contrast injection confirmed the location and the wire was coiled within the gallbladder. The cystic duct was then dilated with 6–8 or 10 Fr dilating catheter (Soehendra; Cook Endoscopy, Winston-Salem, NC) over the guidewire. A transcystic double-pigtail plastic stent was placed (5, 7, or 10 Fr), crossing the ampulla with a proximal and distal pigtail in the gallbladder and duodenum, respectively, in order to decompress the gallbladder.
Endoscopic ultrasound-guided gallbladder drainage
All procedures were performed under monitored or general anesthesia by an experienced endosonographer. The gallbladder was identified using a linear EUS (GF-UCT180; Olympus) and examined to ensure adequate proximity to either the duodenal or gastric wall. Color flow Doppler imaging was used to identify regional vasculature. The puncture site was located either in the duodenal bulb or in the pre-pyloric antrum of the stomach. A 19-gage needle (EchoTip Ultra Endoscopic Ultrasound Needle; Cook Endoscopy) was used to puncture the luminal wall and advance into the gallbladder under both ultrasound and fluoroscopic guidance. Bile was aspirated and sent for culture. A 0.035 guidewire was advanced through the needle and coiled into the gallbladder. When a non-cautery tip LAMS (AXIOS™, Boston Scientific) was placed, a 6 Fr biliary dilating catheter (Soehendra Biliary Dilatation Catheter; Cook Endoscopy) and/or a 4-mm dilating balloon (Hurricane Balloon; Boston Scientific) were used to dilate the transluminal tract; delivery system of the LAMS was then advanced through the fistula tract. If a cautery-tipped LAMS was placed, then the delivery system was inserted in one step into the gallbladder lumen without prior dilation, either over the wire or freehand. The LAMS was deployed under endosonographic and direct endoscopic guidance, with the distal phalange in the gallbladder and the proximal phalange in the enteral lumen (Fig. 1A, B). Once the LAMS was deployed, the gallbladder was dilated to 10 or 15 mm diameter to permit optimal drainage at the discretion of the endoscopist (Fig. 1C, D).
Panel of endoscopic ultrasound-guided transmural gallbladder drainage using lumen-apposing metal stents figures. A Endosonographic vision of deployment of the internal flange of the LAMS into the gallbladder. B Endoscopic visualization of a 15 mm LAMS. C Fluoroscopic view of Dilation of LAMS to 15 mm after deployment into the gallbladder. D Endoscopic visualization of a 15 mm LAMS dilated to its full diameter with a balloon
Postprocedural follow-up
All patients were monitored post-procedure, and their diets were advanced as tolerated. Effective gallbladder decompression and drainage was defined as (1) Clinically symptomatic resolution, (2) Temperature to < 99 °F, and (3) Reduction in white cell counts to < 10 × 103/mm within 72 h of drainage [19]. The patients were discharged home if they were asymptomatic and afebrile. If the patients did not have resolution of their symptoms alongside persistent fevers, they were evaluated for procedural adverse events or ongoing acute cholecystitis.
Long-term patient follow-up
Only patients with a follow-up of ≥ 3 months were included in the study. The decision to proceed to eventual surgical cholecystectomy was based upon the decision of the surgeon and patient.
Patients in the PGBD group had a tube cholecystogram 8 weeks after the initial procedure to evaluate cystic duct patency. If the cystic duct was found to be patent, the cholecystostomy tubes were either removed or plugged and left in situ. If the cystic duct was obstructed, then the tube was upsized and long-term cholecystostomy tube drainage to a bag was left.
Patients in the TP group were followed clinically at regular intervals without scheduled stent exchanges. A repeat ERCP for stent management was only performed in cases where there was evidence of stent occlusion.
For patients in the EGBD group, if the patient was eventually deemed a surgical candidate, the LAMS was removed only after 3 months in order to minimize the risk of recurrent cholecystitis and bile leakage. The LAMS was left in place indefinitely if the patient was non-surgical candidate as a result of their poor clinical condition of the patient and/or patients’ refusal.
Outcome variables
Patient demographic variables were collected. All patients underwent a radiographic study (abdominal ultrasound or CT) to distinguish calculous versus acalculous cholecystitis. Aspects of the patient’s hospitalization, including length of stay date of procedure, were recorded. Details of the intervention, number of sessions, and technical success of the procedures were evaluated. The clinical outcomes and hospital course variables were included, such as clinical resolution of cholecystitis, time to clinical resolution, post-procedure pain, and adverse events. The long-term clinical outcomes regarding adverse events need for repeat intervention, and length of follow-up were recorded.
Statistical analysis
This was a retrospective study. The sample size was based on availability of data. All statistical analyses were conducted using STATA 13.0 (StataCorp LP, College Station, TX). Descriptive analyses were presented as percentages, means, and medians as appropriate. ANOVA analysis was conducted for continuous variables and Chi-square was conducted for nominal variables. Logistic regression was conducted to evaluate predictors for clinical success and long-term complications. Statistical significance was determined a priori at p ≤ 0.05.
Results
Baseline characteristics (Table 1)
We evaluated 372 patients with acute cholecystitis who were non-operative candidates (146 PTGBD, 124 TP, and 102 EGBD). The mean age of the patients was 63 years (range 20–99) and 62% were male. Most of the patients presented with calculous cholecystitis (69.4%). A total of 49 (13.3%) patients had an underlying malignancy. All patients had an ASA classification of IV or V. There were no significant differences in the background demographic details between the three groups, except for the patients in EGBD group being significantly older.
Drainage procedures
In the PTGBD group, 59 patients had their gallbladders drained transperitoneally and 87 patients underwent a transhepatic approach.
In the TP group, the sizes of the transpapillary plastic double-pigtail stents were 5 Fr (n = 3), 7 Fr (n = 98), 8.5 Fr (n = 3), and 10 Fr (n = 16).
In the EGBD group, the gallbladder was drained from the gastric antrum in 39 patients, from the first part of the duodenum in 61 patients, and from the jejunum in two patients with previous gastric surgery. A 10 × 10 mm LAMS was placed in 78 cases and a 15 × 10 mm in 24 patients.
The numbers of procedures performed in each center are summarized in Table 2.
Technical and clinical success (Fig. 2; Table 3)
Technical success of transpapillary stenting for gallbladder drainage (109/124) was significantly lower compared to those who underwent drainage with percutaneous cholecystostomy tube (143/146) and EGBD using LAMS (96/102) [88% vs. 98% vs. 94%, respectively; p = 0.0035]. Failure of transpapillary stenting was as a result of failed cannulation of the bile duct (n = 1), cystic duct obstruction (n = 9), or tortuosity (n = 5). Failure of percutaneous cholecystostomy tube was due to a small gallbladder lumen filled with stones, which did not accommodate the pigtail catheter (n = 3). All six cases of failed EGBD using LAMS were because an enteral puncture gallbladder site could not be identified.
Clinical success with resolution of acute cholecystitis was significantly lower in the TP group (99/124) compared to those who underwent drainage via a PTGBD (141/146) and EGBD using LAMS (92/102) [80% vs. 97% vs. 90%, respectively; p < 0.001]. The median number of procedures required for clinical success was statistically significantly higher in PTGBD compared to both EGBD and TP groups (2 vs. 1 vs. 1, p < 0.001).
Procedural adverse events (Table 3)
Procedural adverse events (AEs) occurred in 6 (4.1%) patients in the PTGBD, 9 (7.2%) patients in the TP group and 12 (11.8%) patients in the EGBD group. Even though the EGBD had a higher number of overall procedure-related AEs, this did not reach statistical significance when compared to the other groups (0.07). There were no procedure-related deaths in any group.
Procedural AEs in the PTGBD group included self-limited bleeding (3), infection (1), and bile leak (2) that was treated conservatively with antibiotics and resolved. Procedural AEs in the TP included self-limited bleeding (2), post-ERCP pancreatitis (3), and abdominal pain requiring prolonged observation (4). In the EGBD, procedural AEs included self-limited bleeding (5), self-limited infection at the LAMS implant site in the stomach (1), bile leak (2) that was effectively treated with antibiotics, abdominal pain (2), and luminal perforation after LAM placement because the distal flange had migrated outside the gallbladder (2); these patients underwent emergent surgery and recovered.
We sub-classified procedural adverse events into minor and moderate-severe. Mild AEs were self-limited bleeding, infection (not including sepsis), and abdominal pain. Moderate-severe AEs were gut perforation, bile leak, and acute pancreatitis. There was no statistically significant difference in the severity of procedural AEs between the three groups (p = 0.85).
The mean hospital stay of patients in the EGBD group was significantly less compared to TP and PTGBD (16 vs. 18 vs. 19 days; p = 0.01).
Late adverse events (Table 4)
Late-term adverse events were less frequent in the EGBD as compared to the TP and the PTGBD groups [2 (1.9%) vs. 6 (4.8%); vs 29 (19.8%); p < 0.001]. Patients in the EGBD and TP group required fewer unplanned hospital readmissions as compared to the PTGBD group (4% vs. 3.2% vs. 19.8%; p < 0.001); admissions in the PTGBD group were predominantly due to problems related to the cholecystostomy tube (Table 4).
In the PTGBD group, long-term adverse events included catheter dislodgement (n = 2), significant abdominal pain at catheter site (n = 2), recurrent cholecystitis due to catheter occlusion (n = 4), cellulitis (n = 5), wound infection with peritubal leak (n = 5), and an intra-abdominal abscess (n = 2). All these patients had one or more unplanned admissions due to cholecystostomy tube that required reintervention (Table 5).
In the EGBD group, one patient had recurrent cholecystitis that resolved with conservative treatment alone. One patient had spontaneous distal migration of the LAMS into the stomach; the stent was removed endoscopically and substituted with another LAMS stent.
In the TP group, two patients had distal stent migration into the bowel requiring a repeat ERCP with replacement of the stents. Four patients had stent occlusion leading to recurrent cholecystitis; the stents were removed endoscopically and substituted with another double-pigtail plastic stent.
Patient follow-up
The median duration of follow-up was 3 months (range 3–9). 258 (69%) patients were alive at date of last follow-up; there was no difference in patient mortality between the three groups (p = 0.84).
In the PTGBD group, a tube cholecystogram at 8 weeks showed a patent cystic duct in 82 patients (56.1%). These patients had their cholecystostomy tubes removed or plugged with a spigot. No LAMS was removed in the EGBD group. In the TP group, ERCP for stent change was performed in 6 (4.8%) patients as a result of stent migration or occlusion. There was no difference in the procedural outcomes and adverse events in the EGBD group for those patients that had gallbladder drainage via the antrum or duodenum.
Surgical removal of the gallbladder was eventually required in 24% of patients either due to recurrent acute cholecystitis not amenable to non-surgical therapy or when the patient had improved medically. The timing of surgery was between 1 and 4 months after the index intervention. A significantly higher number of patients in the PTGBD group underwent surgical cholecystectomy as compared to the TP and EGBD with LAMS group (49% vs. 11% vs. 4%; p < 0.0001).
In patients that did not require a cholecystectomy, those that underwent TP drainage had a significantly lower clinical success (78%) for resolution of acute cholecystitis as compared to those that underwent PTGBD (94%) or EGBD with LAMS (92%); p = 0.002.
Logistic regression analyses
After controlling for age, gender, pathology, number of sessions, and technical success, only the number of sessions variable (1 or more than 1 session) was a statistically significant predictor of clinical resolution (OR = 0.036, 95% CI 0.004–0. 353, p value = 0.0043). 115 patients underwent more than one session (2–7) out of which 104 (90.4%) belonged to the PTGBD group. This indicates that the clinical resolution odds were higher in patients if they had more than one procedure session during PTGBD as opposed to undergoing only one session in the EGBD and TP groups.
After controlling for age, gender, pathology, number of sessions, and technical success, calculous pathology and number of sessions were statistically significant predictors of adverse events. Patients with calculous pathology were two times likely to experience an adverse event (OR = 1.9, 5% CI 1.04–3.57, p value = 0.04), while patients undergoing more than one procedure session were almost three times likely to experience an adverse event (OR = 2.7, 95% CI 1.23–6.07, p value = 0.0138).
Discussion
Cholecystectomy currently is the mainstay of treatment for acute cholecystitis. However, it can be associated with significant postoperative morbidity and mortality in high-risk surgical [23]. In such cases, PTGBD, ET, or EGBD have been successful as alternative non-surgical therapy.
While the current gold standard for gallbladder drainage in high-risk surgical patients is still a percutaneous cholecystostomy tube, it is still associated with adverse events in up to 25 % of patients, which include bleeding, pneumothorax, pneumoperitoneum, bile leakage, and accidental catheter dislodgement. The percutaneous cholecystostomy tube has other inherent disadvantages, which include tube dislodgement in up to 10% of cases, abdominal pain at the tube site, and need for care of the cholecystostomy tube. Adverse events due to the above-mentioned issues often lead to repeated procedures and unplanned hospital admissions. Additionally, we sometimes encounter patients who are unable to tolerate the percutaneous transhepatic approach (e.g., due to ascites, anticoagulant/antiplatelet therapy, disseminated intravascular coagulation, gallbladder malposition, or severe contracture).
Due to the complications associated with percutaneous cholecystostomy tube drainage, there needs to be an alternative to achieve gallbladder drainage in this subset of patients. Therefore, endoscopic methods of gallbladder drainage have increasingly been employed in larger centers in high-risk patients [23].
Endoscopic drainage of the gallbladder offers internal drainage, which increases patient comfort and negates the complications that result from the external drain. ERCP with transpapillary gallbladder stenting has been proposed as a viable strategy for long-term management of acute cholecystitis in poor surgical candidates [13, 24]. Pannala et al. described 51 patients who underwent ERCP with transpapillary stenting for acute cholecystitis [25]. The technical and clinical success rates of 100% and 98%, respectively, support this therapy’s utility for the treatment of cholecystitis in patients who are poor surgical candidates. Lee et al. showed that 80% of patients that underwent TP for gallbladder drainage were able to maintain stent patency without requiring stent exchange for at least 2 years. The transpapillary stent in these situations acts as a “wick” with bile flowing around the stent, so long-term stent patency itself is not imperative to maintaining bile flow from the gallbladder. Despite the relative success of transpapillary gallbladder stenting, it has some limitations and risks. Cystic duct perforation has been described, although this is rare and can be managed conservatively in most cases [26]. TP is also a technically challenging procedure, requiring a skilled endoscopist to negotiate the cystic duct in patients with acute cholecystitis. This is often difficult as a result of inflammatory strictures, tumor involvement, stones, or a tortuous duct.
EGBD can be a safe and minimally invasive alternative approach to surgery in selected patients with acute cholecystitis. EUS-guided gallbladder drainage using plastic stents have resulted in limited gallbladder decompression along with the risk of bile or intestinal content leakage into the peritoneum. Uses of conventional tubular SEMS might lead to migration and biliary leak [27]. LAMS avoids these drawbacks by means of distal anchor flanges that ensure both lumen apposition and drainage. Studies where LAMS have been utilized for gallbladder drainage have reported with few minor acute complications and high clinical success (90–100%) [28].
The current study is the largest to data comparing PTGBD with TP and EGBD in the non-surgical patients with acute cholecystitis. Although this was a retrospective cohort study, all three populations were of similar demographics, baseline clinical severity, and a similar length of follow-up, except for the patients in EGBD group being older.
PTGBD was superior to EGBD with LAMS and to TP in terms of clinical success. However, PTGBD group had to undergo more than one session (2–7) to achieve clinical resolution as opposed to EGBD and TP requiring only one session for clinical resolution. More than one session also significantly increased the odds of adverse events.. EGBD was also associated with a significantly shorter length of hospitalization compared to the other two groups. EGBD via placement of LAMS placement was used in our patient population as a definitive treatment of acute cholecystitis. The majority of these patients did not require removal or revision of the LAMS, hence leading to fewer sessions than PTGBD and TP. It should be noted that the median duration of follow-up in the current study was short (3 months). This was based upon multiple variables including its retrospective nature and the fact that these patients had multiple comorbidities and/or an underlying malignancy that may have caused death within the follow-up period.
In our patients, the percutaneous drains were removed after 8 weeks of placement if the cystic duct was found to be patent, so as to improve patient comfort and reduce the risk of infection and fistula formation. However, multiple sessions in these patients were frequently required because of catheter clogging, infection, or migration. In our study, 77 (52.7%) patients with PGBD procedures required more than two sessions before completion of therapy. In comparison, repeat procedure for stent management (migration or occlusion) was required in only 4.8% patients in the TP group and 1.9% of patients in the EGBD group. This underlies the fact that internal gallbladder drainage using transmural LAMS placement can provide permanent and definite therapy for acute cholecystitis in this relatively sick subset of patients.
Although the procedure-related adverse events (short term) were higher in the EGBD group (11.8%) as compared to patients who underwent PTGBD (4.1%) or TP (7.2%) drainage, this difference was not statistically significant (p = 0.07). No procedure-related deaths were recognized. Procedural AEs may have been higher in the EGBD group related to more preexistent comorbidities in that group. In the PGBD group, there were two patients with bile leak and one with bacteremia after drain placement, which were treated with antibiotics and resolved; there were three patients with bleeding that was controlled with conservative management. In the TP group, patients had self-limited bleeding at the sphincterotomy site that was controlled endoscopically (n = 2), mild post-ERCP pancreatitis (n = 3), and self-limited abdominal pain (n = 4). Patients that underwent EGBD with LAMS had self-limited bleeding at the stent site after placement in five patients, infection (n = 1) and bile leak (n = 2) that were controlled with intravenous antibiotics, and two cases of luminal perforation as a result of stent maldeployment by the endoscopist that had to undergo emergent surgery.
PTGBD had significantly more long-term adverse events compared to EGBD and TP. The majority of adverse events in the PTGBD group were due to catheter-related problems, including catheter dislodgement, catheter occlusion, and skin or intra-abdominal infections at the catheter site. These adverse events required repeat intervention and unplanned hospital admissions in many cases.
There are several recent trials that have evaluated the use of lumen-apposing and biflanged metal stents for EUS-guided gallbladder drainage [29,30,31,32]. These studies have shown that EUS-guided gallbladder drainage is safe and efficacious, with comparable technical and clinical success rates and no difference in adverse events when compared to percutaneous drains. These stents are superior to plastic stents when used for transmural gallbladder drainage because they are fully covered to reduce bile leakage, their design allows promotes direct organ lumen apposition, and have an anti-migratory property. The stents used in these studies were the AXIOS or SPAXUS (Niti-S; Taewoong, Ilsan, South Korea) stents [19, 28, 33, 34]. These findings are also supported by a recent systemic review that concluded that among the different drainage approaches in the non-surgical management of acute cholecystitis, EUS-guided transmural stenting using LAMS for gallbladder drainage appears to be feasible, safe, and effective [35].
Not only can the EUS-guided procedure using LAMS allow for gallbladder drainage, but it can also allow gallstones to be removed through the stent, thus avoiding stone impaction into the stent and reduce the risk of cholecystitis recurrence. In addition, stones from the gallbladder can be removed after LAMS placement [32], In addition, as demonstrated in the present study, LAMS provided definitive treatment for acute cholecystitis for high-risk surgical patients for the majority of the patients. Follow-up in patients with LAMS showed an extremely low incidence of long-term adverse events. We advocate that the type of therapy used for gallbladder decompression in non-surgical candidates should be individualized. For example, a percutaneous gallbladder drain may be best when therapeutic endosonography is not available or the patient is too debilitated to undergo anesthesia, while TP can be reserved for those with pending liver transplants, large amount of ascites or significant coagulopathy. EUS-guided gallbladder drainage with LAMS should be preferred for most patients with malignancy or comorbidities when therapeutic endosonography is available.
The current study has several limitations. Firstly, its retrospective design. Secondly, there may have been a lack of standardization in the patient selection criteria type of the procedure chosen between different centers, and procedure and operator variation at each center. Lastly, only 2 out of 7 centers reported PTGBD data, due to predominance of the interventional radiology for GBD at the time of data collection. These centers have recently started the slow and steady transition to EGBD or TP. However, This study confirms a recent meta-analysis recently published and represents the largest and only reported study to date comparing these three modalities for gallbladder drainage for acute cholecystitis in non-surgical patients [29]. In addition, we have not accounted for variations in patient outcomes and adverse events between the different centers.
In conclusion, EGBD is superior to TP in efficacy but similar to PTGBD. However, EGBD has less overall adverse event rate and number of unplanned admissions. Almost half of patients with percutaneous drains required additional surgical intervention to achieve resolution of acute cholecystitis, while EGBD can be offered as a definitive procedure. We strongly advocate that EGBD should be performed by experienced interventional endoscopists and that caution should be exercised in institutions that are less experienced in this procedure.
References
Hirota M, Takada T, Kawarada Y et al (2007) Diagnostic criteria and severity assessment of acute cholecystitis: Tokyo Guidelines. J Hepato-Biliary-Pancreat Surg 14:78–82
Houghton PWJ, Jenkinson LR, Donaldson LA (1985) Cholecystectomy in the elderly: a prospective study. Br J Surg 72:220–222
Patterson EJ, McLoughlin RF, Mathieson JF et al (1996) An alternative approach to acute cholecystitis. Surg Endosc 10:1185–1188
Schlenker C, Trotter JF, Shah RJ et al (2006) Endoscopic gallbladder stent placement for treatment of symptomatic cholelithiasis in patients with end-stage liver disease. Am J Gastroenterol 101:278–283
Shrestha R, Trouillot TE, Everson GT (1999) Endoscopic stenting of the gallbladder for symptomatic gallbladder disease in patients with end-stage liver disease awaiting orthotopic liver transplantation. Liver Transplant Surg 5:275–281
Kjaer D, Kruse A, Funch-Jensen P (2007) Endoscopic gallbladder drainage of patients with acute cholecystitis. Endoscopy 39:304–308
Chopra S, Dodd GD, Mumbower AL et al (2001) Treatment of acute cholecystitis in non-critically ill patients at high surgical risk. Am J Roentgenol 176:1025–1031
Ito K, Fujita N, Noda Y et al (2004) Percutaneous cholecystostomy versus gallbladder aspiration for acute cholecystitis: a prospective randomized controlled trial. Am J Roentgenol 183:193–196
Elmunzer BJ, Novelli PM, Taylor JR et al (2011) Percutaneous cholecystostomy as a bridge to definitive endoscopic gallbladder stent placement. Clin Gastroenterol Hepatol 9:18–20
Frazee RC, Nagorney DM, Mucha P (1989) Acute acalculous cholecystitis. Mayo Clin Proc 64:163–167
McGahan JP, Walter JP (1985) Diagnostic percutaneous aspiration of the gallbladder. Radiology 155:619–622
Toyota N, Takada T, Amano H et al (2006) Endoscopic naso-gallbladder drainage in the treatment of acute cholecystitis: alleviates inflammation and fixes operator’s aim during early laparoscopic cholecystectomy. J Hepato-Biliary-Pancreat Surg 13:80–85
Lee T, Park D, Lee S et al (2011) Outcomes of endoscopic transpapillary gallbladder stenting for symptomatic gallbladder diseases: a multicenter prospective follow-up study. Endoscopy 43:702–708
Itoi T, Sofuni A, Itokawa F et al (2008) Endoscopic transpapillary gallbladder drainage in patients with acute cholecystitis in whom percutaneous transhepatic approach is contraindicated or anatomically impossible. Gastrointest Endosc 68:455–460
Conway JD, Russo MW, Shrestha R (2005) Endoscopic stent insertion into the gallbladder for symptomatic gallbladder disease in patients with end-stage liver disease. Gastrointest Endosc 61:32–36
Barkay O, Bucksot L, Sherman S (2009) Endoscopic transpapillary gallbladder drainage with the SpyGlass cholangiopancreatoscopy system. Gastrointest Endosc 70:1039–1040
Gaglio PJ, Buniak B, Leevy CB (1996) Primary endoscopic retrograde cholecystoendoprosthesis: a nonsurgical modality for symptomatic cholelithiasis in cirrhotic patients. Gastrointest Endosc 44:339–342
Kedia P, Sharaiha RZ, Kumta NA et al (2015) Endoscopic gallbladder drainage compared with percutaneous drainage. Gastrointest Endosc 82:1031–1036
Teoh AY, Serna C, Penas I et al (2017) Endoscopic ultrasound-guided gallbladder drainage reduces adverse events compared with percutaneous cholecystostomy in patients who are unfit for cholecystectomy. Endoscopy 49:130–138
Cotton PB, Eisen GM, Aabakken L et al (2010) A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc 71:446–454
Yokoe M, Takada T, Strasberg SM et al (2012) New diagnostic criteria and severity assessment of acute cholecystitis in revised Tokyo Guidelines. J Hepatobiliary Pancreat Sci 19:578–585
Massoumi RL, Trevino CM, Webb TP (2016) Postoperative complications of laparoscopic cholecystectomy for acute cholecystitis: a comparison to the ACS-NSQIP risk calculator and the Tokyo guidelines. World J Surg 41(4):935–939
Aranha GV, Sontag SJ, Greenlee HB (1982) Cholecystectomy in cirrhotic patients: a formidable operation. Am J Surg 143:55–60
Tamada K, Seki H, Sato K et al (1991) Efficacy of endoscopic retrograde cholecystoendoprosthesis (ERCCE) for cholecystitis. Endoscopy 23:2–3
Pannala R, Petersen BT, Gostout CJ et al (2008) Endoscopic transpapillary gallbladder drainage: 10-year single center experience. Minerva Gastroenterol Dietol 54:107–113
Gosain S, Bonatti H, Smith L et al (2010) Gallbladder stent placement for prevention of cholecystitis in patients receiving covered metal stent for malignant obstructive jaundice: a feasibility study. Dig Dis Sci 55:2406–2411
Kahaleh M, Perez-Miranda M, Artifon EL et al (2016) International collaborative study on EUS-guided gallbladder drainage: are we ready for prime time? Dig Liver Dis 48(9):1054–1057
Itoi T, Binmoeller KF, Shah J et al (2012) Clinical evaluation of a novel lumen-apposing metal stent for endosonography-guided pancreatic pseudocyst and gallbladder drainage (with videos). Gastrointest Endosc 75:870–876
Khan MA, Atiq O, Kubiliun N et al (2017) Efficacy and safety of endoscopic gallbladder drainage in acute cholecystitis: is it better than percutaneous gallbladder drainage? Gastrointest Endosc 85(1):76–87. e3–e32
Irani S, Ngamruengphong S, Teoh A et al (2017) Similar efficacies of endoscopic ultrasound gallbladder drainage with a lumen-apposing metal stent versus percutaneous transhepatic gallbladder drainage for acute cholecystitis. Clin Gastroenterol Hepatol 15(5):738–745
Tyberg A, Saumoy M, Sequeiros EV et al (2018) EUS-guided versus percutaneous gallbladder drainage: isn’t it time to convert? J Clin Gastroenterol 52(1):79–84
Chan SM, Teoh AYB, Yip HC et al (2017) Feasibility of per-oral cholecystoscopy and advanced gallbladder interventions after EUS-guided gallbladder stenting. Gastrointest Endosc 85(6):1225–1232
Moon JH, Choi HJ, Kim DC et al (2014) A newly designed fully covered metal stent for lumen apposition in EUS-guided drainage and access: a feasibility study (with videos). Gastrointest Endosc 79:990–995
Walter D, Teoh AY, Itoi T et al (2016) EUS-guided gall bladder drainage with a lumen-apposing metal stent: a prospective long-term evaluation. Gut 65:6–8
Anderloni A, Buda A, Vieceli F et al (2016) Endoscopic ultrasound-guided transmural stenting for gallbladder drainage in high-risk patients with acute cholecystitis: a systematic review and pooled analysis. Surg Endosc 30(12):5200–5208
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Michel Kahaleh, MD has received grant support from Boston Scientific, Fujinon, EMcision, Xlumena Inc., W.L. Gore, MaunaKea, Apollo Endosurgery, Cook Endoscopy, ASPIRE Bariatrics, GI Dynamics, NinePoint Medical, Merit Medical, Olympus, and MI Tech. He is a consultant for Boston Scientific, Xlumena Inc., Concordia Laboratories Inc, AbbVie, and MaunaKea Tech. Ali Siddiqui, MD is a consultant for Boston Scientific, Cook Endoscopy, and Medtronic. He has received research grant support from Boston Scientific and Medtronic. He is a speaker for AbbVie. Jose Nieto, MD is a consultant for Boston Scientific. Shawn Mallery, MD is a consultant for Boston Scientific. Douglas Adler, MD is a consultant for Boston Scientific. Thomas Kowalski, MD is a consultant for Boston Scientific and Medtronic. David Loren, MD is a consultant for Boston Scientific and has received grant support from Medtronic. Rastislav Kunda, MD is a consultant for Boston Scientific, BCM Korea, Olympus Japan, and Omega Medical Imaging. Eric Chrisiansen MD, Monica Gaidhane MD, Amy Tyberg MD, Usama Iqbal MD, Tayebah Mumtaz MD, Arish Noor MD, Mustafa Arain MD, and Monica Saumoy MD, have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Siddiqui, A., Kunda, R., Tyberg, A. et al. Three-way comparative study of endoscopic ultrasound-guided transmural gallbladder drainage using lumen-apposing metal stents versus endoscopic transpapillary drainage versus percutaneous cholecystostomy for gallbladder drainage in high-risk surgical patients with acute cholecystitis: clinical outcomes and success in an International, Multicenter Study. Surg Endosc 33, 1260–1270 (2019). https://doi.org/10.1007/s00464-018-6406-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-6406-7