Abstract
Background
Gastroduodenostomy is preferred as a method of reconstruction following distal subtotal gastrectomy. However, in initial reports on reduced-port gastrectomy, gastroduodenostomy has rarely been performed therein because of technical difficulties. The present study describes a novel intracorporeal gastroduodenostomy technique applicable during reduced-port robotic distal subtotal gastrectomy.
Methods
Data were retrospectively reviewed for cases of reduced-port (three-port) robotic distal subtotal gastrectomy with intracorporeal delta-shaped gastroduodenostomy performed from February 2016 to December 2016. The reduced-port approach used a Single-Site™ port via a 25-mm infraumbilical incision and two additional ports. We performed intracorporeal gastroduodenostomy using a 45-mm robotic or laparoscopic endolinear stapler. All staplers were inserted via a port on the left lower abdomen.
Results
In our initial experience with intracorporeal gastroduodenostomy, 28 consecutive patients underwent successful surgery with the technique without needing to convert to open, laparoscopic, or conventional five-port robotic surgery. Mean operation time was 201.1 min (110–282 min), and no major complications, including anastomosis-related problems, were recorded.
Conclusions
Intracorporeal delta-shaped gastroduodenostomy was safely and feasibly applied during reduced-port robotic gastrectomy with acceptable operative outcomes and no major complications. Intracorporeal gastroduodenostomy should be considered during reduced-port distal subtotal gastrectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Minimally invasive surgery is now a standard option for the treatment of gastric cancer. Efforts to minimize trauma, surgical stress, and scarring after surgery have drawn interest to reduced-port gastrectomy among laparoscopic surgeons. Although the safety and feasibility of reduced-port laparoscopic or robotic gastrectomy for gastric cancer have been reported by experienced surgeons, [1, 2] intracorporeal anastomosis remains a challenge because of restricted access.
Intracorporeal gastroduodenostomy, also known as delta-shaped anastomosis, during laparoscopic distal subtotal gastrectomy was first reported by Kanaya [3]. Originally, achieving anastomosis with this technique required five ports for adequate access and support from assistants. Nonetheless, even during conventional five-port gastrectomy, intracorporeal gastroduodenostomy poses greater technical challenges than other types of anastomoses, such as loop or Roux-en-Y gastrojejunostomy, and, thus, has rarely been performed, despite being preferred in conventional laparoscopic surgery [2, 4,5,6,7,8,9,10,11].
In this study, we describe a novel, safe, and easy intracorporeal delta-shaped gastroduodenostomy technique applicable during reduced-port (three-port) robotic gastrectomy using the da Vinci® surgical system (Intuitive Surgical, Sunnyvale, CA, USA).
Patient and methods
We conducted a single-center, retrospective review of data on gastric cancer with reduced-port robotic distal subtotal gastrectomy and intracorporeal delta-shaped gastroduodenostomy using endolinear stapling devices. From February 2016 to December 2016, 28 consecutive patients who underwent this procedure with a curative aim were included in this study. All patients provided written informed consent for the procedure. If minimally invasive surgery was indicated, as described in a previous study, [12] eligible patients were provided detailed descriptions of each procedure, including reduced-port robotic or five-port conventional laparoscopic gastrectomy. Patients could then choose between these options. All patients who chose robotic gastrectomy consecutively underwent reduced-port gastrectomy using a Single-Site™ port (Intuitive Surgical, Sunnyvale, CA, USA) and two additional ports during the study period. Delta-shaped gastroduodenostomy was performed as the primary option for reconstruction whenever possible. When gastroduodenostomy was not feasible, gastrojejunostomy was performed. Approval was obtained from the Institutional Review Board of Severance Hospital, Yonsei University College of Medicine (4-2017-0533).
Technique
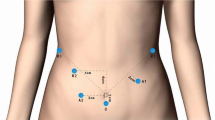
Reduced-port robotic distal subtotal gastrectomies were performed using both the da Vinci® Si and Xi Surgical Systems. The reduced-port approach uses a Single-Site™ port positioned below the umbilicus and two additional straight cannulas. For the procedure, a camera was introduced through the endoscope cannula of the Single-Site™ port, and the two additional cannulas were inserted in the abdomen, one on each side: an 8-mm cannula on the right upper abdomen and a 12-mm cannula on the left lower abdomen. Then, curved and assist cannulas were inserted via the Single-Site™ port. To perform lymph node dissection, we placed ultrasonic shears (Harmonic®, Ethicon Endo-surgery, Cincinnati, OH, USA) in the right abdominal cannula, Maryland bipolar forceps (Intuitive Surgical, Sunnyvale, CA, USA) in the left abdominal cannula, and 5-mm semi-rigid Cadiere forceps (Intuitive Surgical, Sunnyvale, CA, USA) in the curved cannula of the Single-Site™ port (Fig. 1).
For intestinal resection and anastomosis, the ultrasonic shears equipped via the right abdominal cannula were removed and replaced with rigid Maryland bipolar forceps for retraction of the stomach. Cadiere forceps inserted through the curved cannula of the Single-Site™ port were also used for retraction. To begin intracorporeal gastroduodenostomy, the bulb of the duodenum was transected in the dorso-ventral direction with a 45-mm endolinear stapler inserted through the left lower abdominal port (Fig. 2A). Coordinating the Maryland forceps and the stapler, duodenum was easily rotated and transected. After D1+ or D2 lymphadenectomy (according to clinical stage) and mobilization of the stomach were performed, the stomach was transected in the usual fashion (Fig. 2B), and the resected specimen was put into a plastic bag. Intracorporeal delta-shaped gastroduodenostomy was then created using an EndoWrist® stapler 45 (Intuitive Surgical, Sunnyvale, CA, USA) or a 45-mm laparoscopic flexible endolinear stapler (Echelon Flex™ Ethicon Endo-Surgery, Cincinnati, OH, USA). When using a laparoscopic endolinear stapler, an 8-mm straight cannula was inserted in a port-in-port fashion via the 12-mm trocar (XCEL®, Ethicon Endo-surgery, Cincinnati, OH, USA) in the left lower abdomen. Small incisions for anastomosis were created along each edge of the stomach and the duodenum. A 45-mm endolinear stapler was then inserted via the left abdominal cannula into each hole at the stomach and the duodenum. Gastroduodenal continuity was achieved by firing the first stapler in the posterior walls of the remnant stomach and the duodenum (Fig. 2C). A V-shaped anastomosis was created on the posterior walls, and the entry hole was closed using one or two 45-mm endolinear staplers (Fig. 2D–F). The video supplement (Supplemental Digital Content 1) for the procedure exhibits intracorporeal delta-shape gastroduodenostomy using endolinear staplers during reduced-port robotic distal subtotal gastrectomy for gastric cancer.
Gastric resection and intracorporeal delta-shaped gastroduodenostomy during reduced-port robotic distal subtotal gastrectomy. During anastomosis, rigid Maryland bipolar forceps are introduced through the right abdominal cannula to retract the duodenum and stomach in coordination with 5-mm semi-rigid Cadiere forceps inserted through the curved cannula in the Single-Site™ port. A The duodenum is transected in the dorso-ventral (posterior to anterior) direction. B The stomach is resected from the greater curvature to the lesser curvature using two or three 45-mm endolinear staplers through the left lower cannula. C The posterior wall of the remnant stomach and the duodenum are approximated using a 45-mm endolinear stapler via the left lower abdominal port. D For closure of the common entry hole, the staple line is retracted using curved Cadiere forceps and Maryland forceps. E Closure of the entry hole with a 45-mm endolinear stapler. F Final closure of the entry hole and completion of the gastroduodenostomy
Results
During the study period, reduced-port robotic distal subtotal gastrectomy was performed for 32 consecutive patients. Among them, 28 patients underwent intracorporeal delta-shaped gastroduodenostomy. All robotic gastrectomies were successfully completed without converting to open or laparoscopic surgery or additional port insertion. The preoperative characteristics of the patients including 15 males and 13 females are shown in Table 1. Mean age and body mass index were 52.9 years (range of 30–82 years) and 23.5 kg/m2 (range of 17.0–29.8 kg/m2), respectively. Clinical T1 cancer was suspected in 17 (60.7%) patients, and 11 (39.3%) patients were diagnosed as having advanced gastric cancer preoperatively. Table 2 outlines the operative parameters and postoperative results. To achieve gastroduodenal anastomosis, laparoscopic endolinear staplers were used in 15 patients (53.6%), and robot staplers were utilized in 13 patients (46.4%). The total operation time for reduced-port robotic gastrectomy was 201.1 min (range of 110–282 min). Blood loss was estimated at 43.6 mL (range of 10–120 mL). The mean number of retrieved lymph nodes was 60.7 (range of 26–119). The mean postoperative hospital stay was 6.1 days (range of 5–14 days). Assessed by the Clavien–Dindo system, neither major (grade III or higher) nor anastomosis-related complications were recorded during the study period. Also, there were no instances of readmission or reoperation for any surgery-related cause among the enrolled patients with at least 6 months of follow-up.
Discussion
In this study, we described a novel anastomosis technique for completing intracorporeal delta-shaped gastroduodenostomy during reduced-port robotic gastrectomy. In our initial experience therewith, we have found the short-term surgical outcomes of the procedure, including operating time, hospital stay, and postoperative complications, to be acceptable, comparable with those in previous studies on conventional minimally invasive gastrectomy. During the follow-up period of the present study, no major complications, including anastomosis-related problems, were recorded. These results suggest that intracorporeal gastroduodenostomy may be feasible and safe in reduced-port robotic gastrectomy.
According to reports, gastroduodenostomy is physiologically advantageous in terms of maintaining duodenal passage, nutrient uptake, and iron metabolism [13, 14]. Additionally, postoperative ileus and obstruction have been found to be less frequent after gastroduodenostomy than after other anastomoses after distal subtotal gastrectomy [15]. Moreover, gastroduodenostomy allows for the same access to the papilla of Vater and the biliary system as with endoscopy after surgery. With these advantages, gastroduodenostomy has been most commonly used for conventional open and minimally invasive distal subtotal gastrectomies [16]. Our first choice for gastric reconstruction after distal subtotal gastrectomy during minimal invasive surgery is also intracorporeal delta-shaped gastroduodenostomy: in cases where the lesion is close to the pylorus of the stomach or small remnant stomach tissue is left due to tumor location, gastrojejunostomy is preferred to gastroduodenostomy.
Intracorporeal gastroduodenostomy in reduced-port gastrectomy is difficult to perform when using conventional devices and techniques. Unlike conventional minimally invasive gastrectomy that typically utilizes five ports, reduced-port gastrectomy requires ports to be placed in a position that creates an angle inadequate for applying endolinear staplers. Moreover, because of constricted angles and a fewer number of ports, instrument collision occurs more frequently, and traction is much more difficult. Thus, for reduced-port surgery, teamwork between the surgeon and assistants is very important, and an expert surgical team with extensive experience is desirable. For these reasons, loop or Roux-en-Y gastrojejunostomy has been preferred above gastroduodenostomy in most previous studies of reduced-port gastrectomy. According to a nationwide survey by the Korean Gastric Cancer Association, gastroduodenostomy is not commonly performed after single or reduced-port distal subtotal gastrectomy (reportedly in only 21.5%) [17]. As shown in Table 3, in a small case series, the proportion of gastroduodenostomies undertaken was much lower for reduced-port gastrectomies than for conventional gastrectomies performed by the same author. With reference to the results of multicenter prospective randomized controlled trials in Korea and Japan, the proportions of gastroduodenostomies after laparoscopic distal subtotal gastrectomy were 64.1 and 64.6%, respectively [10, 11].
Meanwhile, we have found that the intracorporeal gastroduodenostomy method described in the current study is technically feasible and safe to perform during reduced-port robotic gastrectomy. During anastomosis, adequate traction was obtained using the arms of the robotic system without difficulty. Also, the procedure was somewhat familiar to the surgeon because the staplers were inserted in the same direction as in conventional intracorporeal delta-shape gastroduodenostomy during minimally invasive gastrectomy. Moreover, when using the robot stapler, almost no help from the assistant was needed. Although the laparoscopic endolinear staplers were sufficient for gastroduodenostomy in reduced-port robotic surgery using the technique described herein, they did require greater skill from the assistants, relative to use of the robot stapler. The robot stapler provided wider articulation, with a range of 108° side-to-side, while a laparoscopic stapler can be somewhat limited in articulation. Moreover, positioning, griping, clamping, and firing of the robot stapler can be fully controlled by the operator in the console. Thus, use of the robot stapler could enable surgeons to perform intracorporeal anastomosis fully without the help of a well-trained assistant surgeon.
Nevertheless, this retrospective analysis has inherent limitations. The results of this study represent the initial experience of a single surgeon. Comparative studies of short-term outcomes of reduced-port intracorporeal gastroduodenostomy in regards to patient benefits, such as operative outcomes, recovery, and surgical stress after surgery, should be conducted. Also, future studies regarding safety and nutritional benefit of reduced-port intracorporeal gastroduodenostomy in comparison to other reconstruction methods should follow. Additionally, research on the cost effectiveness and efficacy of the robot stapler compared with laparoscopic endolinear staplers is necessary.
Conclusion
In this study, we described a technique for completing intracorporeal gastroduodenostomy during reduced-port robotic distal subtotal gastrectomy for gastric cancer and demonstrated the short-term surgical outcomes thereof. Reduced-port intracorporeal delta-shaped gastroduodenostomy was easily implemented and could be safely and feasibly applied using a current robotic surgical system with acceptable operative outcomes. Intracorporeal gastroduodenostomy should receive greater consideration for patients undergoing reduced-port distal subtotal gastrectomy.
References
Omori T, Oyama T, Akamatsu H, Tori M, Ueshima S, Nishida T (2011) Transumbilical single-incision laparoscopic distal gastrectomy for early gastric cancer. Surg Endosc 25(7):2400–2404
Lee S, Kim JK, Kim YN, Jang DS, Kim YM, Son T, Hyung WJ, Kim HI (2017) Safety and feasibility of reduced-port robotic distal gastrectomy for gastric cancer: a phase I/II clinical trial. Surg Endosc 31(10):4002–4009
Kanaya S, Gomi T, Momoi H, Tamaki N, Isobe H, Katayama T, Wada Y, Ohtoshi M (2002) Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy: new technique of intraabdominal gastroduodenostomy. J Am Coll Surg 195(2):284–287
Ahn SH, Son SY, Jung DH, Park DJ, Kim HH (2014) Pure single-port laparoscopic distal gastrectomy for early gastric cancer: comparative study with multi-port laparoscopic distal gastrectomy. J Am Coll Surg 219(5):933–943
Usui S, Tashiro M, Haruki S, Matsumoto A (2014) Triple-incision laparoscopic distal gastrectomy for the resection of gastric cancer: comparison with conventional laparoscopy-assisted distal gastrectomy. Asian J Endosc Surg 7(3):197–205
Kashiwagi H, Kumagai K, Monma E, Nozue M (2015) Dual-port distal gastrectomy for the early gastric cancer. Surg Endosc 29(6):1321–1326
Kim SM, Ha MH, Seo JE, Kim JE, Choi MG, Sohn TS, Bae JM, Kim S, Lee JH (2015) Comparison of reduced port totally laparoscopic distal gastrectomy (duet TLDG) and conventional laparoscopic-assisted distal gastrectomy. Ann Surg Oncol 22(8):2567–2572
Shibao K, Sato N, Higure A, Yamaguchi K (2015) A new oval multichannel port to facilitate reduced port distal gastrectomy. Minim Invasive Ther Allied Technol 24(3):135–140
Lee CM, Park DW, Park S, Kim JH, Park SH, Kim CS (2017) Lymph node dissection using bipolar vessel-sealing device during reduced port laparoscopic distal gastrectomy for gastric cancer: result of a pilot study from a single institute. J Laparoendosc Adv Surg Tech A 27(11):1101–1108
Kim W, Kim HH, Han SU, Kim MC, Hyung WJ, Ryu SW, Cho GS, Kim CY, Yang HK, Park DJ, Song KY, Lee SI, Ryu SY, Lee JH, Lee HJ, Korean Laparo-endoscopic Gastrointestinal Surgery Study G (2016) Decreased morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage I gastric cancer: short-term outcomes from a multicenter randomized controlled trial (KLASS-01). Ann Surg 263(1):28–35
Katai H, Mizusawa J, Katayama H, Takagi M, Yoshikawa T, Fukagawa T, Terashima M, Misawa K, Teshima S, Koeda K, Nunobe S, Fukushima N, Yasuda T, Asao Y, Fujiwara Y, Sasako M (2017) Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric Cancer 20(4):699–708
Obama K, Kim YM, Kang DR, Son T, Kim HI, Noh SH, Hyung WJ (2017) Long-term oncologic outcomes of robotic gastrectomy for gastric cancer compared with laparoscopic gastrectomy. Gastric Cancer 21(2):285–295
Lee JH, Hyung WJ, Kim HI, Kim YM, Son T, Okumura N, Hu Y, Kim CB, Noh SH (2013) Method of reconstruction governs iron metabolism after gastrectomy for patients with gastric cancer. Ann Surg 258(6):964–969
Kim BJ, O’Connell T (1999) Gastroduodenostomy after gastric resection for cancer. Am Surg 65(10):905–907
Park DJ, Lee HJ, Kim HH, Yang HK, Lee KU, Choe KJ (2005) Predictors of operative morbidity and mortality in gastric cancer surgery. Br J Surg 92(9):1099–1102
Kumagai K, Shimizu K, Yokoyama N, Aida S, Arima S, Aikou T, Japanese Society for the Study of Postoperative Morbidity after G (2012) Questionnaire survey regarding the current status and controversial issues concerning reconstruction after gastrectomy in Japan. Surg Today 42(5):411–418
Information Committee of Korean Gastric Cancer A (2016) Korean gastric cancer association nationwide survey on gastric cancer in 2014. J Gastric Cancer 16(3):131–140
Acknowledgements
This study was supported by a faculty research grant from Yonsei University College of Medicine (6-2016-0109).
Author information
Authors and Affiliations
Contributions
JHL and TS designed the study; JHL, JK, WJS, CKR, and TS collected all materials; JHL, JK, and TS drafted the paper; and MC, HIK, and WJH critically revised subsequent drafts. All authors have read and approved the submission manuscript.
Corresponding author
Ethics declarations
Disclosures
Drs. Chul Kyu Rho, Hyoung-Il Kim, Jisu Kim, Joong Ho Lee, Minah Cho, Taeil Son, Won Jun Seo, and Woo Jin Hyung have no conflicts of interest or financial ties to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Digital Content 1.mp4: Representative view of intracorporeal delta-shaped gastroduodenostomy using endolinear staplers during reduced-port robotic distal subtotal gastrectomy. (MP4 254934 KB)
Rights and permissions
About this article
Cite this article
Lee, J.H., Son, T., Kim, J. et al. Intracorporeal delta-shaped gastroduodenostomy in reduced-port robotic distal subtotal gastrectomy: technical aspects and short-term outcomes. Surg Endosc 32, 4344–4350 (2018). https://doi.org/10.1007/s00464-018-6244-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-6244-7