Abstract
Background
Laparoscopic techniques in colorectal surgery have been widely utilised due to short-term patient benefits but conversion to open surgery is associated with adverse short- and long-term patient outcomes. The aim of this study was to investigate the influence of dual specialist operating on the conversion rate and patient outcomes following laparoscopic colorectal surgery.
Methods
A prospectively populated colorectal cancer surgery database was reviewed. Cases were grouped into single or dual consultant procedures. Cluster analysis and odds ratio (OR) were used to identify risk factors for conversion. Primary outcome measures were conversion to open and five year overall survival (OS) calculated using the Kaplan–Meier log-rank method.
Results
750 patients underwent laparoscopic colorectal cancer resection between 2002 and 2015 (median age 73, 319 (42.5%) female, 282 (37.6%) rectal malignancies, 135 patients (18%) had two consultants). The single surgeon conversion rate was 20.4% compared to 5.5% for dual operating (OR 4.4, 95% CI 1.87–10.2, p < 0.001). There were no demographic or tumour differences between the laparoscopic/converted and number of surgeon groups. Two-step cluster analysis identified cluster I (lower risk) 406 patients, 8% converted and cluster II (higher risk) 261 patients, conversion rate 30%. Median follow-up was 48 months (range 0–168). Five-year OS was significantly inferior for both converted and single surgeon cases (63% vs. 77%, p < 0.001 and 61% vs. 70%, p = 0.033, respectively).
Conclusion
In selected colorectal cancer patients operated by fully trained laparoscopic surgeons, we observed a reduction in conversion with associated long-term survival benefit from dual operating specialists.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The use of minimally invasive techniques for colorectal cancer resection benefits short-term patient recovery with comparable long-term oncological outcomes [1,2,3]. A widespread uptake of laparoscopy and willingness to attempt complex cases has resulted. However, laparoscopic colorectal surgery (LCS) requires advanced skills and is acknowledged to hold a high learning curve. 152 and 143 cases have been suggested as a threshold for competency with a reduction in conversion to open surgery and complications respectively at this point [4].
Although widely reported by surgical studies, the definition of conversion within the literature varies and rates often rely upon surgical honesty. Conversion to open surgery can remove the short-term recovery benefits of a minimal access approach [5] and of clear concern, meta-analysis has shown an increase in local recurrence, short- and long-term mortality following colorectal cancer resection [6]. Strategies to reduce conversion rates are therefore strongly indicated. Initial reports from the ROLAR study did not show a reduction in conversion when novel technology was utilised [7] but other potential strategies have not been explored.
When training, mentored cases are associated with a lower conversion rate when compared with independent cases [8] and no differences in conversion and post-operative clinical outcomes were observed between expert performed and mentored training cases [3, 8]. It is unknown if this beneficial effect is applicable to the specialist (consultant) setting and whether the presence of two specialists operating on laparoscopic colorectal cases can influence conversion rates and hence the subsequent patient outcome. Therefore, the aim of this study was to investigate the impact of dual specialist operating upon conversion rate, short- and long-term outcomes following LCS.
Methods
An observational review of a dedicated, prospectively compiled colorectal cancer patient database managed by a specialist information analyst team was performed. Initial database creation and review of already held, anonymised data was approved by our local research ethics and data governance committees. Study inclusion criteria were laparoscopic surgical resection, with curative intent, for biopsy-proven or radiological or endoscopic suspicion of colorectal adenocarcinoma between 2002 and 2015. Emergency resections were excluded. No hand-assisted or hybrid laparoscopic procedures were performed. Since 2002, all patients were managed within a previously described enhanced recovery programme [9,10,11] and cared for by a specialist multi-disciplinary team. Conversion to open was defined by our institution as the inability to complete the dissection laparoscopically (including the vascular ligation) and/or requiring an incision larger than that required to remove the specimen [12, 13]. Primary reasons for conversion were prospectively captured and subdivided into proactive (anticipation of difficulty or failure to progress) or reactive (in response to an adverse event) groups.
Yeovil District Hospital served as a national training centre in the LapCo UK national training programme [14] and all four consultants contributing to this study were independent, fully trained surgeons with at least 200 independent laparoscopic colorectal procedures. Dual operating was defined as the planned presence of two consultant colorectal surgeons in theatre and actively involved in the procedure compared to standard practice of a single consultant surgeon operating with a trainee. Where non-colorectal consultants were involved in operations this was not considered as dual operating. Based upon our initial experiences of dual operating with perceived advantages, the model of two consultant colorectal surgeons was incorporated in job plans allowing advance scheduling. The role of the second consultant was pragmatic and varied depending on the level of difficulty of each case from assisting in certain parts of the operation to acting as lead surgeon, allowing the primary surgeon the opportunity for a break during long cases.
Patient demography including age, gender, body mass index (BMI), previous surgery, neoadjuvant therapy as well as pathologically defined tumour staging data were recorded. Length of stay (LoS) was calculated as number of hospital nights stay until discharge to home, rehabilitation or care facility as appropriate with the day of surgery designated day zero. Readmission was defined as unplanned hospital readmission within 30 days. All patients entered a standard clinical, radiological and endoscopic follow-up programme until 5 years post-surgery or death. This manuscript has been designed in accordance with the STROBE guidelines [15].
Statistical analysis
The data were analysed using SPSS (IBM SPSS Statistics v24, USA) and STATA (v11, StataCorp Texas, USA) for uni, multivariate and survival analyses. For categorical data, analysis included the use of cross tabulation, odds ratios and chi-squared to test the difference or association between groups. Fisher’s exact test was used when indicated. The Pearson chi-squared test of association was used to examine the relationship between each variable and outcome. The magnitude of the effect was quantified using the odds ratio (OR) with 95% confidence interval. Data are displayed as medians with interquartile and overall ranges unless specified. T-test and Mann–Whitney U were used to compare medians from normal and non-normally distributed populations, respectively.
To guide future decision making, two-step cluster analysis was performed to divide the cohort into low- and high-risk groups to identify pre-operative demographic and tumour risk factors associated with conversion. The Kaplan–Meier log-rank method was used to estimate 5-year overall survival (OS) outcomes. A p value of < 0.05 was considered significant.
Results
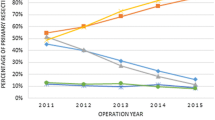
750 consecutive patients met inclusion criteria (median age 73 [25–96], 319 (42.5%) females, 282 (37.6%) rectal malignancies, Table 1). Median follow-up was 48 months (range 0–168). 616 operations (82%) were performed by a single consultant with 134 cases (18%) operated by dual colorectal consultants. There were no differences in T, N, M or stage between one consultant and dual surgeon cases (p = 0.329, 0.372, 0.844 and 0.627, respectively). The overall conversion rate was 16.9% (n = 127, Table 2) and did not vary across the study timeframe (p = 0.109, Supplementary Fig. 1) or between colonic and rectal cases (16.3% vs. 17.5%, p = 0.701). No differences in tumour data were observed between the laparoscopic and converted groups (p = 0.088, Table 1). The conversion rate for those who were operated on by a single consultant was 20.4% compared to 5.5% for dual operating surgeons (OR 4.4, 95% CI 1.87–10.2, p < 0.001, Table 2).
The majority of conversions took place for proactive reasons (88%). Commonly, adverse tumour factors such as local invasion or perforation (32%), adhesions (25%) and patient factors such as high body mass index (BMI) or narrow pelvic inlet (18%). Reactive conversion due to adverse events was encountered in 15 patients (12% of conversions and 2% overall).
Cluster analysis was carried out on 667 patients as 83 (11.1%) cases were excluded due to incomplete data. 406 patients were identified as low risk (cluster I, 7.6% converted, Table 3) with 261 patients classified as high risk for conversion (cluster II, 29.9%). No demographic differences were seen between the two clusters including histopathology defined TNM tumour data (Table 1). Rectal cancer (OR 2.3, 95% CI 1.1–4.9, p = 0.003) and neoadjuvant therapy (OR 3, 95% CI 1.7–5.4, p = 0.006) were identified as risk factors for conversion. Operative times (203 (88) min vs. 235 (98) min, p = 0.004), blood loss (137 ml vs. 186 ml, p = 0.013) and stoma formation were significantly higher in cluster II (53% vs. 24%, p = 0.004, Table 1). Fewer dual operating consultant cases were seen in cluster II (19.7% vs. 15.1%, p = 0.06, Table 3). LoS and 30-day readmission were significantly higher in cluster II (9 vs. 7, p = 0.001; 16.3%, vs 13.8%, p = 0.005).
For the entire cohort and both the laparoscopic and converted patient groups, OS was seen to follow tumour stage results with a non-significant lower OS observed in higher stage cancers (Supplementary Fig. 2a–c, p = 0.062 and p = 0.105, respectively). OS did not differ between study surgeons (p = 0.676).
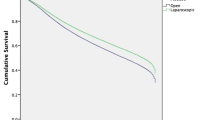
In those converted, OS was significantly inferior compared to those receiving a laparoscopic completed procedure (63% vs. 77%, p < 0.001, Fig. 1). There was also a significant improvement in 5-year OS observed between those who were operated with two consultants (70% (95% CI 69–85) vs. 61% (54–67), p = 0.033, Fig. 2).
Discussion
Despite the advancement in LCS techniques, technology, training and experience, the conversion rate after colorectal cancer surgery remains variable and in certain cases, concerningly high [6]. Conversion negatively impacts on short- and long-term outcomes making this a potential target for quality improvement with direct translational patient benefits [6]. This study was designed to investigate whether there was any advantage to dual consultant operating with particular focus on conversion and long-term survival.
To our knowledge, this study is the first to report on this method of colorectal cancer surgical service delivery. Whilst our overall conversion rate is comparable with previous reports [6, 16, 17], we observed a four-fold conversion increase in single surgeon cases, compared with dual consultant procedures, despite no patient demographic or tumour stage differences between the groups. Overall survival was significantly inferior for patients either operated by a single consultant or converted to open surgery. We observed few adverse events requiring immediate laparotomy, as 88% of conversions were proactive, most commonly tumour or adhesion related. We can hypothesise that the second consultant provided sufficient support at critical steps allowing safe continuation of laparoscopic procedures and therefore avoiding the negative patient outcomes associated with conversion.
Although many surgeons already support each other during complex cases, this often remains an informal practice. In our institution, two operating consultants were possible through job plan restructuring following the initial perceived positive impact of this strategy. The role of the second surgeon was often pragmatic and based upon pre-operative case assessment and on table progress.
Deciding which patients would benefit most from dual specialists is crucial as health services are unlikely to support dual surgeon operating for every LCS case. Therefore we performed cluster analysis modelling to guide future practice by identifying conversion risk factors that may benefit from dual consultant operating. Our model identified the known challenges and current areas of controversy in LCS of rectal cancer [18, 19], neoadjuvant therapy and requiring stoma formation as predictors for conversion. These patients may benefit most from a dual operating approach. Interestingly, in contrast to previous reports, we observed that age, gender, ASA, BMI and tumour location were not predictors for conversion in our cohort [16]. One possible explanation is that dual surgeons may help to overcome these traditional surgical difficulties.
Two specialist operators are commonplace in comparable high-risk industries such as commercial aviation where standard practice mandates both a pilot and a co-pilot with clearly defined roles [20]. In our centre, the second surgeon’s role was flexible in response to each case. In addition to providing support and assisting decision-making processes, the second surgeon could also lead surgery and allow the primary surgeon the opportunity for a break during long cases. Prolonged surgery can present physical, ergonomic and visual challenges and is associated with inferior outcomes [12] whereas surgeons report focus and performance improvements when short breaks are taken [21, 22]. We observed that a common role for the second consultant was to assist the lead surgeon in difficult parts of the pelvic dissection and act as a “navigator” particularly when planes were obscured by adhesions, radiotherapy and/or an advanced tumour. Dual operating is likely to promote good team working practices and continuing professional development. The second consultant can gain detailed knowledge of the patient and their procedure which may potentially foster post-operative patient care benefits particularly where multisite or shift working is employed.
There are few previous reports on dual specialist operating, all coming from complex procedures in other surgical disciplines [23,24,25]. Spinal deformity surgeons subjectively reported dual operating improved safety, decreased complications and improved outcomes. However, financial considerations limited widespread uptake of this strategy [23]. During bilateral breast cancer surgery, dual operating reduced theatre time by 35% [24]. Two surgeon liver resection resulted in lower blood loss and a shorter LoS [25]. We are unaware of any prior reports on dual specialist operating in laparoscopic surgery or long-term patient outcome data.
Future directions of study should focus upon the global impact of dual specialist operators, including economic and human factors. The health care economics of this practice has not been evaluated and we understand that this approach may have an implication on service provision, particularly in the private sector. However, we propose dual consultant operators only in selected cases and predominantly, the second consultant was only required for 1–2 h during the critical phase of the procedure. Job plan restructuring was necessary to ensure availability of the second surgeon but this was still only possible in 18% of patients. The associated cost implication may be offset by reduced conversions and their resulting benefits particularly a shorter LoS. Whilst the presence of a physically and mentally fresh surgeon for complex parts of a procedure appears to be a logical one, further research is required to define the mechanism behind the observed benefits. Future studies should investigate factors known to influence patient outcomes such as specimen quality, lymph node yield and resection margin status. Capturing intraoperative events including performance would also help to explore these core considerations. Finally, although a trainee was always present during dual procedures and frequently the additional consultant continued to teach whilst working with the first surgeon, the impact upon training has not been explored and focused assessment of this issue is indicated.
Whilst we present a large mature cohort of consecutive patients from a well-established LCS training centre with experienced surgeons, this study has a number of limitations. The finding that conversion correlated with long-term survival may not imply a causative association and should be interpreted with caution as it was not possible to control for the large number of confounding factors that can influence this result. Although we used histopathological results for tumour staging, this would not be available to inform pre-operative decisions. Clinical staging, disease-free survival and specimen quality data are of interest but was not routinely captured in these patients. Our subjective risk stratification did not correlate with the cluster analysis findings and further research is required to validate and refine this model. Nevertheless, we still achieved a four-fold reduction in the conversion rate for those patients who were operated on by dual consultants. Extrapolating this result, if dual operating surgeons were applied to the whole high-risk cluster, a further 60 patients may have avoided conversion.
Conclusion
In selected colorectal cancer patients operated by fully trained laparoscopic surgeons, we observe a reduction in conversion with associated long-term survival benefit from dual operating specialists.
References
Spanjersberg WR, van Sambeeck JD, Bremers A, Rosman C, van Laarhoven CJ (2015) Systematic review and meta-analysis for laparoscopic versus open colon surgery with or without an ERAS programme. Surg Endosc 29(12):3443–3453
Kuhry E, Schwenk WF, Gaupset R, Romild U, Bonjer HJ (2008) Long-term results of laparoscopic colorectal cancer resection. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003432.pub2
Currie AC, White I, Malietzis G, Moorghen M, Jenkins JT, Kennedy RH (2016) Outcomes following laparoscopic rectal cancer resection by supervised trainees. Br J Surg 103(8):1076–1083
Miskovic D, Ni M, Wyles SM, Tekkis P, Hanna GB (2012) Learning curve and case selection in laparoscopic colorectal surgery: systematic review and international multicenter analysis of 4852 cases. Dis Colon Rectum 55(12):1300–1310
Allaix ME, Furnee EJ, Mistrangelo M, Arezzo A, Morino M (2016) Conversion of laparoscopic colorectal resection for cancer: what is the impact on short-term outcomes and survival? World J Gastroenterol 22(37):8304–8313
Clancy C, O’Leary DP, Burke JP, Redmond HP, Coffey JC, Kerin MJ et al (2015) A meta-analysis to determine the oncological implications of conversion in laparoscopic colorectal cancer surgery. Colorectal Dis 17(6):482–490
Collinson FJ, Jayne DG, Pigazzi A, Tsang C, Barrie JM, Edlin R et al (2012) An international, multicentre, prospective, randomised, controlled, unblinded, parallel-group trial of robotic-assisted versus standard laparoscopic surgery for the curative treatment of rectal cancer. Int J Colorectal Dis 27(2):233–241
Miskovic D, Wyles SM, Ni M, Darzi AW, Hanna GB (2010) Systematic review on mentoring and simulation in laparoscopic colorectal surgery. Ann Surg 252(6):943–951
Boulind CE, Yeo M, Burkill C, Witt A, James E, Ewings P et al (2012) Factors predicting deviation from an enhanced recovery programme and delayed discharge after laparoscopic colorectal surgery. Colorectal Dis 14(3):e103–e110
King PM, Blazeby JM, Ewings P, Franks PJ, Longman RJ, Kendrick AH et al (2006) Randomized clinical trial comparing laparoscopic and open surgery for colorectal cancer within an enhanced recovery programme. Br J Surg 93(3):300–308
Smart NJ, White P, Allison AS, Ockrim JB, Kennedy RH, Francis NK (2012) Deviation and failure of enhanced recovery after surgery following laparoscopic colorectal surgery: early prediction model. Colorectal Dis 14(10):e727–e734
Harrison OJ, Smart NJ, White P, Brigic A, Carlisle ER, Allison AS et al. Operative time and outcome of enhanced recovery after surgery after laparoscopic colorectal surgery. JSLS 2014;18(2):265–72
Kennedy RH, Francis EA, Wharton R, Blazeby JM, Quirke P, West NP et al (2014) Multicenter randomized controlled trial of conventional versus laparoscopic surgery for colorectal cancer within an enhanced recovery programme: EnROL. J Clin Oncol 32(17):1804–1811
Coleman MG, Hanna GB, Kennedy R (2011) The National Training Programme for Laparoscopic Colorectal Surgery in England: a new training paradigm. Colorectal Dis 13(6):614–616
von EE, Altman, Egger DG, Pocock M, Gotzsche SJ, Vandenbroucke PC (2007) JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 4(10):e296
Thorpe H, Jayne DG, Guillou PJ, Quirke P, Copeland J, Brown JM (2008) Patient factors influencing conversion from laparoscopically assisted to open surgery for colorectal cancer. Br J Surg 95(2):199–205
Healthcare Quality Improvement Partnership. UK National Bowel Cancer Audit Annual Report 2016. 2017. NHS Digital. 3-5-2017
Web References
Stevenson AR, Solomon MJ, Lumley JW, Hewett P, Clouston AD, Gebski VJ et al (2015) Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA 314(13):1356–1363
Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas M et al (2015) Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA 314(13):1346–1355
Jimenez C, Kaspar K, Rivera J, Talone A, Jentsch F (2016) Crew resource management. In: Proceedings of the human factors and ergonomics society annual meeting, vol 59, pp 946–950
Hallbeck MS, Lowndes BR, Bingener J, Abdelrahman AM, Yu D, Bartley A et al (2017) The impact of intraoperative microbreaks with exercises on surgeons: A multi-center cohort study. Appl Ergon 60:334–341
Park AE, Zahiri HR, Hallbeck MS, Augenstein V, Sutton E, Yu D et al (2017) Intraoperative “micro breaks” with targeted stretching enhance surgeon physical function and mental focus: a multicenter cohort study. Ann Surg 265(2):340–346
Scheer JK, Hey L, LaGrone M, Daubs M, Ames CP (2016) 343 results of the 2015 SRS survey on single versus two attending surgeon approach for adult spinal deformity surgery. Neurosurgery 63(Suppl 1):201
Mallory MA, Losk K, Camuso K, Caterson S, Nimbkar S, Golshan M (2016) Does “two is better than one” apply to surgeons? Comparing single-surgeon versus co-surgeon bilateral mastectomies. Ann Surg Oncol 23(4):1111–1116
Yamada N, Amano R, Kimura K, Murata A, Yashiro M, Tanaka S et al (2015) Two-surgeon technique for liver transection using precoagulation by a soft-coagulation system and ultrasonic dissection. Hepatogastroenterology 62(138):389–392
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Prof. Francis, Mr. Curtis, Dr. Crilly, Miss. Noble, Dr. Dyke, Mr. Hipkiss, Mr. Dalton, Mr. Allison, Dr. Salib and Mr. Ockrim confirm they have no conflicts of interest or financial ties to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
464_2018_6097_MOESM1_ESM.tif
Supplementary material 1 (TIF 1662 KB) Activity and conversion rate across the study timeframe. No significant difference in conversion rate is seen between years (p = 0.109)
464_2018_6097_MOESM2_ESM.tif
Supplementary material 2 (TIF 1200 KB) Overall survival for the entire cohort displayed by histopathologically determined tumour stage. More advanced tumours are seen to have lower OS (p = 0.062)
464_2018_6097_MOESM3_ESM.tif
Supplementary material 3 (TIF 1191 KB) Overall survival for cases completed laparoscopically displayed by tumour stage. Again, as expected, OS is observed to mirror staging data (p = 0.105)
464_2018_6097_MOESM4_ESM.tif
Supplementary material 4 (TIF 1055 KB) Supplementary Figure 2c - Overall survival for converted cases displayed by tumour stage. Once more long term survival follows tumour staging results (p = 0.105)
Rights and permissions
About this article
Cite this article
Francis, N.K., Curtis, N.J., Crilly, L. et al. Does the number of operating specialists influence the conversion rate and outcomes after laparoscopic colorectal cancer surgery?. Surg Endosc 32, 3652–3658 (2018). https://doi.org/10.1007/s00464-018-6097-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-6097-0