Abstract
Background and objectives
Endoscopic over the scope clip (OTSC) closure represents a new technique for endoscopic management of enteric bleeding and tissue defects such as anastomotic leaks and enterocutaneous (EC) fistulas. We aim to describe our technical approach for OTSC closure of EC fistulas and convey our outcomes.
Methods and procedures
This retrospective review includes ten patients who underwent OTSC application for EC fistulas by surgical endoscopists at a US tertiary care hospital from July 2015 to October 2017. Demographic data, along with type of defect, location, duration of lesion, success or failure of OTSC, and nutritional status were compiled. The ACS NSQIP surgical risk calculator was used to project the risk of mortality, complications, length of stay, and risk of readmission had our patients undergone surgical correction of their fistula.
Results
Overall success for EC fistula closure was 70%. Acute fistulas were closed with a success rate of 86%. Chronic fistulas were closed successfully in only 33% of cases. Of patients successfully closed, NSQIP-predicted rates of mortality, any complication, and median length of stay were 21.1%, 34.5%, and 9.5 days, respectively. With OTSC, these patients experienced 0 mortalities, 0 complications, and had a median length of stay of 4 days.
Conclusion
OTSC is an effective adjunctive measure to improving rates of successful closure of EC fistulas and compromised anastomosis. OTSC conveys a markedly improved procedural risk profile as compared to standard surgical correction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Enterocutaneous (EC) fistulas represent a difficult problem for the surgeon. Historically, the treatment of these fistulas has been intensive medical management with bowel rest, total parenteral nutrition, and subsequent surgical correction for fistulas that fail to close with non-operative management. It is reported that 30–35% of EC fistulas will close with non-operative treatment [1]. The reported mortality rate for this process ranges from 10 to 20% [2]. In addition to being a debilitating, burdensome disease process for the patient, EC fistulas are also economically costly for health care systems. A retrospective review published in 2009 examined the resource utilization of trauma laparotomies complicated by the development of EC fistulas. EC fistula formation significantly increased ICU length of stay (LOS), hospital LOS, and over all increased the hospital charges on the order of four hundred thousand dollars per patient [3].
In cases of non-resolving fistulas, surgical intervention represents a potentially perilous endeavor as the abdomen can be hostile. Operative intervention demands extensive lysis of adhesions, bowel resection, and complex abdominal wall reconstruction [1, 2]. The first endoscopic clips to enhance our armamentarium were introduced in the early 1990s. While useful for small defects, they proved to be less useful for larger defects because of restricted opening distance between jaws, low closure force, and inability to capture deep tissue [4]. With the advent of the over the scope clip (OTSC; Ovesco Endoscopy AG, Tubingen, Germany), the surgical endoscopist now has an additional option for treatment.
The OTSC system seeks to produce a more durable closure of visceral defects by including the entire thickness of the bowel wall while also applying a greater compressive force of 8–9 N [5]. It uses a super elastic, biocompatible, nitinol clip which is pre-loaded on an applicator tip mounted to the tip of most endoscopes [4,5,6]. OTSC can be used for multiple purposes including EC fistulas, acute bleeding, anastomotic leaks, NOTES surgery, and closure of acute perforations [4,5,6].
In this study, we will seek to describe our experience with the OTSC system in regard to EC fistulas alone. We will describe the technique in detail and our outcomes thus far.
Materials and methods
Patients
This is a retrospective, single-institution, IRB-approved study performed at the University of South Alabama from July 2015 until October 2017. All patients undergoing OTSC application to EC fistula were included. General demographic indicators including age and sex were obtained. The nutritional state of our patients was analyzed by using albumin and pre-albumin as adjunct markers. Total procedural time was recorded. The location and acuity of the fistulas were tabulated. Fistulas present for ≤ 30 days were considered acute, while fistulas > 30 days were considered chronic. Technical success was defined as lack of intraluminal dye following OTSC clip application. Clinical success was defined as a closed fistula tract 1 month following application. The American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) Surgical Risk Calculator (SRC) was used to assess the anticipated surgical morbidity and mortality of our subjects had they undergone primary surgical repair [7, 8]. CPT code 44640 was used, along with patient characteristics, to determine surgical mortality, risk of any complication, predicted LOS, and predicted rate of readmission. These risk profiles were obtained as a surrogate marker for the overall clinical status of our patients as possible surgical candidates. Using NSQIP data for our patients with successful OTSC placement, we compared predicted surgical outcomes with outcomes following OTSC.
Indications and timing of intervention
To undergo OTSC, fistulas had to be accessible by endoscopy and patients’ physiology had to allow for procedural sedation. In the setting of an acutely developing fistula, we advocate for immediate intervention as soon as the patient is stable enough to undergo endoscopic exploration. In the setting of an established fistula, timing of intervention can be delayed to allow for extended perioperative optimization.
OTSC system
The OTSC system consists of three main components. The first component is a handwheel, which is attached to the working channel of the endoscope and secured in place with a Velcro strap. The next component is a thread retriever. The thread retriever is passed through the working channel to the tip of the endoscope. The final component is an application cap and pre-mounted clip with an attached thread. The thread retriever is used to pull the delivery thread through the working channel of the endoscope. The thread is then attached to the handwheel. The application cap is placed on the tip of the endoscope. The endoscopist is able to deploy the clip by turning the handwheel.
Clip selection
The type of clip should be selected based on lesion characteristics, desired endoscope, and anastomotic location. There are three diameters available: 11 mm, 12 mm, and 14 mm. The selection of clip diameter is based upon both the lesion size and the available endoscope. In each size there are two depths available: 4 mm, and 6 mm. In the setting of fistulas, we always select the 6 mm depth as to incorporate a judicious volume of tissue. Finally, there are three types of clips currently available. Type “A” has blunt teeth and gives a compressive effect and is useful for bleeding. Type “T” has teeth with small spikes and allows for both compression and anchoring. Type “T” clips are generally selected for fistulas distal to the stomach, unless the lesion is particularly edematous or harbors a significant amount of inflammation at the time of intervention. Type “GC” has elongated teeth with spikes and was designed primarily for gastric closures. We have used the GC clip on gastric, small bowel, and colonic fistulas as it incorporates well into inflamed and fibrotic tissue.
Endoscopic technique
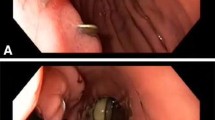
We begin our procedure by endoscopically identifying the fistulous tract. After noting the suspected lesion, we confirm its location by flushing normal saline and then dilute methylene blue (Fig. 1). After the intraluminal fistulous defect is identified, we remove the endoscope, assemble the OTSC system as described, and reinsert the scope to the internal opening of the fistula. There are multiple ways in which to incorporate the fistula into the OTSC applicator tip prior to clip deployment. One can attempt to use suction alone, a basic grasping instrument, or one of multiple specially designed instruments offered by Ovesco™. When the defect and surrounding visceral wall is within the applicator cap, the handwheel is turned, thus deploying the clip. We then visualize the deployed clip and repeat the injection of dilute methylene blue into the cutaneous fistula tract opening. Cessation of flow of methylene blue into the lumen of the bowel portends fistula source closure and technical success. Persistence of flow affords a treatment challenge.
Results
Baseline characteristics
Over a 27-month period, ten patients met inclusion criteria. Acute fistulas predominated our cohort (n = 7, 70%), while chronic fistulas were less commonly seen (n = 3, 30%). Table 1 summarizes the patient characteristics of our patients with EC fistulas. Of the 10 patients, 5 (50%) were male and 5 (50%) were female. The average age at time of intervention was 54.5 (SE ± 3.9, range 32–76). Nutritional status was assessed using laboratory data. The mean albumin of our study population was 2.6, SEM ± 0.23 (range 1.6–3.7) (normal reference range 3.4–5), while the mean pre-albumin value was 14.1, SEM ± 1.1, (range 8.7–18.6) (normal reference range 18–35.7). None of our patients had undergone prior abdominal or pelvic radiation. Table 1 summarizes the patient characteristics of those with EC fistula. Median procedural time was found to be 51 min. ACS NSQIP SRC for our cohort, if they had undergone operative correction, showed the median risk of mortality was 0.95% (0.1–16.7%), while the median risk of any complication was 37.3% (20–64.2%). The median risk for readmission was calculated to be 19.5% (16–32.6%). The median estimated LOS for our cohort, if surgical correction had been pursued, was 9.8 days (7–22 days). Table 2 summarizes the NSQIP-predicted surgical outcomes for all patients.
Outcomes
Technical success was achieved in 90% (9/10) of OTSC applications, with no procedural mortality or morbidity. The overall clinical success rate for closure of fistulas was 70% (7/10). Closure of acute fistulas, ≤ 30 days old, resulted in an 86% (6/7) success rate, while closure of chronic fistulas, > 30 days old, resulted in a 33% (1/3) success rate. The success rate for females was 100% (5/5) and 40% (2/5) for males. There were no mortalities or operative complications associated with OTSC application in our cohort. In our patients with successful OTSC placement, the median NSQIP SRC-predicted perioperative mortality, median predicted rate of any complication, and median predicted LOS was 1.1%, 34.5%, and 9.5 days, respectively. These patients’ mortality rate with OTSC was 0%, there was a 0% rate of any complication, and the median LOS after OTSC application was 4 days (Table 3).
Discussion
EC fistulas represent a difficult pathologic entity that conveys significant morbidity and mortality. Surgical intervention is typically difficult and associated with significant complication rates [1, 2]. The evolution of treatment for EC fistulas which do not spontaneously resolve has been slow and the mainstay remains intensive medical management and interval surgical intervention.
Endoscopic intervention with OTSC represents an evolution in the treatment of this disease process that has the potential to broadly impact clinical practice. OTSC is a minimally invasive endoscopic technique which exposes patients to minimal morbidity and can result in significantly reduced duration of disease, decreased duration of hospitalization, and obviate the need for surgical intervention. Additionally, it is our experience that OTSC is a technique that is easily added to the surgical endoscopist’s skill set.
Three entities in the technical profile of OTSC application can prove challenging: localization of the fistula, navigation of the endoscope with the cap in place, and incorporation of chronically inflamed tissue. We have found that injection of the extracorporal fistula tract with normal saline and diluted methylene blue helps significantly with localization and confirmation of the intraluminal component of the fistulous tract. We have not seen this technique described in our literature review. Navigating the endoscope with the application cap in place is difficult as the cap is somewhat bulky and can make endoscopic navigation more difficult. Finally, grasping of tissue prior to clip application is a valuable step of the technique. This can prove quite difficult with either acutely inflamed and edematous mucosa or with chronically fibrotic fistulas. There are multiple modalities to assist with tissue incorporation such as endoscopic suction, basic endoscopic graspers, or graspers specifically designed for use with the OTSC platform.
The majority of literature currently available on OTSC comes from small case series and show mixed data [5, 6, 9,10,11]. Two large multicenter retrospective reviews regarding OTSC outcomes for fistulas have been published, showing an overall success rate of 42.9% and 68% [12, 13]. A recent meta-analysis, which included all types of fistulas undergoing closure with OTSC, showed a mean success rate of 69% [16]. Our results come from a small cohort but are similar to those reported thus far with an overall success rate of 70% [12, 13, 16]. Many of the existing studies contain data from multiple different types of fistulas, anastomotic leaks, bleeding, and other pathologies, thus complicating application of data to EC fistulas alone [5, 6, 9,10,11,12,13,14,15,16]. Our series sought to examine EC fistulas alone.
One of our major findings is the higher rate of success with acute fistulas compared to chronic fistulas (86% vs. 33%). Our outcomes agree with the previously published hypothesis that the lesion characteristics of chronic fistula leads to reduced clinical success of OTSC in such fistulas [6, 16,17,18].
ACS NSQIP SRC data were used as a comparator for our patients’ overall clinical status. Our cohort of patients had significant comorbidities, surgically hostile abdomens, and were generally malnourished. Comparing the NSQIP-projected surgical risk rates with outcomes of our OTSC successes highlights the potential OTSC has to reduce morbidity, mortality, and healthcare utilization related to EC fistulas.
In terms of outcomes data, our study is limited by its retrospective nature and small sample size. Further prospective studies as well as long-term efficacy data are needed.
In conclusion, we feel that OTSC is an easily added component to the surgeon’s armamentarium to treat EC fistulas. Our experience has demonstrated that OTSC of EC fistulas can substantially decrease morbidity, hospitalization time, need for major surgical intervention, and possibly mortality.
References
Fischer JE (2017) The management of enterocutaneous fistulas. In: Cameron JL, Cameron AM (eds), Current surgical therapy. Elsevier, Inc., Philadelphia, pp 140–144
Evenson AR, Fischer JE (2007) Gastrointestinal-cutaneous fistulae. In: Fischer JE, Bland KI (eds) Mastery of surgery. Lippincott Williams & Wilkins, Philadelphia, pp 1401–1407
Teixeira PG, Inaba K, Dubose J, Salim A, Brown C, Rhee P, Browder T, Demetriades D (2009) Enterocutaneous fistula complicating trauma laparotomy: a major resource burden. Am Surg 75:30–32
Banerjee S, Barth BA, Bhat YM, Desilets DJ, Gottlieb KT, Maple JT, Pfau PR, Pleskow DK, Siddiqui UD, Tokar JL, Wang A, Wong LM, Song K, Rodriguez SA (2012) Endoscopic closure devices. Gastrointest Endosc 76:244–251
Kirschniak A, Kratt T, Stuker D, Braun A, Schurr MO, Konigsrainer A (2007) A new endoscopic over-the-scope clip system for treatment of lesions and bleeding in the GI tract: first clinical experiences. Gastrointest Endosc 66:162–167
Kirschniak A, Subotova N, Zieker D, Königsrainer A, Kratt T (2011) The over-the-scope clip (OTSC) for the treatment of gastrointestinal bleeding, perforations, and fistulas. Surg Endosc 25:2901–2905
Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, Cohen ME (2013) Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 217:833–842.e1–3
Lubitz AL, Chan E, Zarif D, Ross H, Philip M, Goldberg AJ, Pitt HA (2017) American College of Surgeons NSQIP risk calculator accuracy for emergent and elective colorectal operations. J Am Coll Surg 225:601–611
Repici A, Arezzo A, De Caro G, Morino M, Pagano N, Rando G, Romeo F, Del Conte G, Danese S, Malesci A (2009) Clinical experience with a new endoscopic over-the-scope clip system for use in the GI tract. Dig Liver Dis 41:406–410
Iacopini F, Di Lorenzo N, Altorio F, Schurr MO, Scozzarro A (2010) Over-the-scope clip closure of two chronic fistulas after gastric band penetration. World J Gastroenterol 16:1665–1669
Weiland T (2012) OTSC System—Update on clinical data. http://ovesco.com/fileadmin/Downloads/OTSC_System_clinical_data_eng_Rev01_2012-10-22.pdf. October 2012; Accessed 15 Mar 2018
Chavez YH, Law JK, Kratt T, Arezzo A, Verra M, Morino M et al (2014) International multicenter experience with an over-the-scope clipping device for endoscopic management of GI defects. Gastrointest Endosc 80:610–622
Baron TH, Song LM, Ross A, Tokar JL, Irani S, Kozarek RA (2012) Use of an over-the-scope clipping device: multicenter retrospective results of the first U.S. experience (with videos). Gastrointest Endosc 76:202–208
Wedi E, Gonzalez S, Menke D, Kruse E, Matthes K, Hochberger J (2016) One hundred and one over-the-scope-clip applications for severe gastrointestinal bleeding, leaks and fistulas. World J Gastroenterol 22(5):1844–1853
Arezzo A, Verra M, Reddavid R, Cravero F, Bonino MA, Morino M (2012) Efficacy of the over-the-scope clip (OTSC) for treatment of colorectal postsurgical leaks and fistulas. Surg Endosc 26:3330–3333
Weiland T, Fehlker M, Gottwald T, Schurr MO (2012) Performance of the OTSC system in the endoscopic closure of gastrointestinal fistulae—a meta-analysis. Minim Invasive Ther Allied Technol 21(4):249–258
Albert JG, Friedrich-Rust M, Woeste G, Strey C, Bechstein WO, Zeuzem S et al (2011) Benefit of a clipping device in use in intestinal bleeding and intestinal leakage. Gastrointest Endosc 74:389–397
von Renteln D, Denzer UW, Schachschal G, Anders M, Groth S, Rösch T (2010) Endoscopic closure of GI fistulae by using an over-the-scope clip (with videos). Gastrointest Endosc 72:1289–1296
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. William Richards received financial support from Torax® Medical for research related to LINX® magnetic esophageal sphincter augmentation. Dr. James Roy, Dr. Kaci Sims, Dr. Paul Rider, Dr. Leander Grimm, and Dr. John Hunter have no conflicts of interest or financial ties to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Roy, J., Sims, K., Rider, P. et al. Endoscopic technique for closure of enterocutaneous fistulas. Surg Endosc 33, 3464–3468 (2019). https://doi.org/10.1007/s00464-018-06646-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-06646-1