Abstract
Background
Although previous studies have reported the possibility of therapeutic ERCP without fluoroscopy, more robust documentation of fluoroscopy-free common bile duct stone (CBDS) clearance is needed. Technically, “digital cholangioscopy” (DCS) may be used to confirm CBDS clearance. We aimed to compare the feasibility, safety, and radiation exposure between patients with CBDS undergoing stone removal by DCS and conventional ERCP (cERCP).
Methods
Fifty (50) consecutive patients with a CBDS size < 15 mm underwent DCS (SpyGlass DS Direct Visualization System, Boston Scientific, Marlboro, MA, USA) between December 2015 and October 2016. Of 202 consecutive patients undergoing cERCP during the same time frame, 50 matched pairs were created using propensity score matching analysis. In the DCS group, patients underwent biliary cannulation and CBDS removal without fluoroscopy followed by DCS to confirm complete CBDS clearance. A final occlusion cholangiogram was performed as the current standard of care to confirm CBDS clearance.
Results
Cannulation success rates were similar between the DCS and cERCP groups (98 vs. 98%). By intention-to-treat analysis, CBDS clearance in the DCS and cERCP groups was not different (90 vs. 98%; p = 0.20, respectively). DCS had successful CBDS removal in 45 cases, whereas 5 (10%) failed for clearance by DCS due to technical limitations. Adverse events were not different between both groups.
Conclusions
In the management of uncomplicated CBDS, our data confirmed the feasibility of DCS for CBDS clearance as it showed efficacy and safety comparable to those of cERCP. Although certain conditions may limit its effectiveness, DCS offers the ability to perform CBDS clearance without the need for fluoroscopy unit and can avoid radiation exposure while ERCP under fluoroscopy remains the current standard of care in patients with CBDS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Endoscopic retrograde cholangiopancreatography (ERCP) is the first-line therapy for common bile duct stones (CBDS). This therapy is standardly performed and is a fluoroscopy-guided technique. Radiation exposure to patients and staff during ERCP is an important health and safety concern [1, 2]. Since cumulative doses of ionizing radiation from multiple procedures carry an increased risk of cancer, the American Society for Gastrointestinal Endoscopy (ASGE) recommends minimizing radiation exposure during ERCP [2].
Radiation-free ERCP is needed in particular settings such as dealing with pregnant and critically ill immobilized patients [3,4,5,6,7,8]. However, the data are limited by small sample size. One important limitation of the procedure without fluoroscopy includes the lack of CBDS-clearance confirmation. Thus, in this setting, biliary stenting is common to ensure drainage, followed by repeat ERCP under fluoroscopy for the final CBDS clearance. Either endoscopic ultrasound (EUS) or intraductal ultrasound (IDUS) has been used to confirm CBDS clearance [9,10,11], but EUS may have limitation due to its inability to distinguish CBDS from air bubbles after sphincterotomy [12].
Direct cholangioscopy is a promising technique to confirm CBDS clearance. The first generation of SpyGlass (the Legacy system, Boston Scientific, Marlboro, MA, USA) was launched in 2007. A previous series of studies demonstrated missed CBDS clearance in one of five pregnant patients (20%) using the Legacy system [5]. The incomplete CBDS clearance could be secondary to the limited visibility of the fiber optic cholangioscope. Compared to the Legacy system, a recent digital cholangioscope provides not only a significant improvement in the image quality but also a much easier setup process so that the system can be ready in 5 min [13]. To date, data on the outcomes of digital cholangioscopy (DCS)-assisted CBDS removal are lacking.
The objectives of our study were as follows: (1) to compare the feasibility and safety of DCS-assisted uncomplicated CBDS removal with conventional ERCP (cERCP) using a propensity score matching analysis and (2) to measure how much radiation exposure to the patients [dose area product (DAP; mGy-cm2)] could be avoided in the DCS group when the exposure amount for the cERCP group was used as the reference.
Materials and methods
Study population
Between December 2015 and October 2016, 50 consecutive adult patients with suspected CBDS with a size < 15 mm in diameter were invited to participate in the prospective study of DCS at our institution. The diagnosis of CBDS was based on clinical presentation (biliary pain and/or abnormal liver function tests) and at least one image study [transabdominal ultrasonography (TUS), computed tomography (CT), magnetic resonance cholangiopancreatography (MRCP), or EUS]. The exclusion criteria were a history of bile duct surgery, bile duct stricture, bile duct tumors, severe comorbid diseases, unstable vital signs including septic cholangitis, pregnancy, coagulopathy and a large CBDS (size ≥ 15 mm) as determined by imaging studies. Patients who provided informed consent followed the procedure protocol of DCS (Fig. 1).
Using our endoscopy databases, all patients with CBDS undergoing cERCP during the same timeframe (n = 202) were retrospectively reviewed. Of those patients, 50 match pairs with a 1:1 matched DCS:cERCP ratio were created using propensity score matching analysis. Potential confounders for calculation, including age, sex, CBDS size, presence of a duodenal diverticulum, and previous sphincterotomy, were selected by experienced endoscopists. Relevant demographic and clinical data, including duodenal diverticulum, previous biliary sphincterotomy, CBDS size based on imaging studies, cannulation techniques, total procedure times, and total radiation doses delivered to patients (DAP; mGy-cm2), were abstracted from medical records. Following the procedures, adverse events and hospitalization were observed.
The study protocol was approved by our local institutional review board. The study was registered at clinicaltrials.gov (NCT02967926).
Procedures
Using a side-viewing duodenoscope (TJF-160R or TJF-Q180V; Olympus, Tokyo, Japan) under conscious sedation (midazolam and meperidine), all procedures were performed by one of the four experienced endoscopists who performed > 300 ERCPs per year and > 20 cholangioscopies under fluoroscopy per year. Advanced endoscopy fellows were involved only during scope positioning and biliary cannulation.
In the cERCP group, the patients were put in prone position using the same fluoroscopy unit. The need for fluoroscopy is only controlled by the attending endoscopist. DAP (mGy-cm2) was documented after the procedure was completed. Prophylactic antibiotics were administered. After identifying the papilla, selective bile duct cannulation was performed using a bendable-tip catheter, either an Ultratome XL (Boston Scientific, Marlboro, MA, USA) or a Swing Tip cannula (Olympus, Tokyo, Japan), and a 0.035-inch guide wire was used for standard cannulation. Advanced techniques for biliary cannulation, such as a double guidewire and/or precut sphincterotomy technique, were performed under fluoroscopy guidance if guidewire-assisted cannulation was not achieved within 10 min. Standard techniques, including cholangiogram, endoscopic sphincterotomy, and stone extraction, were performed as addressed in the literature [14]. A final cholangiogram with a balloon sweep was performed to confirm CBDS clearance.
In the DCS group, patients underwent selective biliary cannulation without fluoroscopy. The primary setting for the fluoroscopic control was set to “disable” to prevent unintentional use of fluoroscopy. Following successful bile duct cannulation confirmed by visible bile in the catheter and by smooth advancement of a guidewire without any resistance, a standard biliary sphincterotomy was performed. If a failed standard cannulation occurred, the double guidewire technique was attempted without the use of fluoroscopy. In such case, after pancreatic duct deep cannulation was confirmed by pancreatic juice aspiration, a guidewire was gently advanced to the main pancreatic duct approximately 7–10 cm beyond the papilla or until resistance was detected. Following the first guidewire placement in the main pancreatic duct, the catheter was exchanged and reloaded with the second guidewire. Selective biliary cannulation was aimed at the 10–11 o’clock position, with a left, upward relation to the first guidewire. After successful biliary cannulation was confirmed by the bile aspiration technique, the first guidewire was removed. If failure occurred during double guidewire cannulation without fluoroscopy, standard or advanced biliary cannulation, including precut under fluoroscopy, was re-attempted step by step under fluoroscopic guidance; this situation was considered failed non-fluoroscopic biliary cannulation within the DCS group. Following successful selective biliary cannulation and sphincterotomy, a balloon catheter was gently advanced over the guidewire 10–15 cm beyond the papilla or until resistance was observed, which implied reaching the hilum. Balloon sweeps were performed and repeated until no stones were retrieved. Then, a DCS system (SpyGlass DS Direct Visualization System, Boston Scientific, Marlboro, MA, USA) was advanced into the CBD to confirm complete CBDS clearance (Fig. 2A). If DCS showed residual CBDS (Fig. 2B), another round of non-fluoroscopic CBDS removal was attempted. This process was repeated until there was no residual stone. A final occlusion cholangiogram under fluoroscopy was performed as the gold standard of care to confirm CBDS clearance. If residual stones were seen during the final cholangiogram, the DCS procedure was converted to a cERCP, and the DCS procedure was considered as failed per protocol.
Post-procedure evaluation
In our protocol, following successful CBDS removal, all patients were hospitalized for clinical observation and able to progressively resume a normal diet within 12–24 h if no adverse events related to the procedures developed. Post-procedure pancreatitis was defined as the presence of pancreatitis-type pain plus a serum amylase level over three times the upper normal limit or pain plus evidence of acute pancreatitis seen on imaging studies (CT or MRI). Bleeding, perforation, and cholangitis were defined as described in the literature [15].
Statistical analysis
To compare patient characteristics and clinical outcomes of the DCS group to those of the matched cERCP group, propensity score matching with a 1:1 matched DCS:cERCP ratio was used to reduce differences in possible confounding factors between groups as follows: age, sex, CBDS size, duodenal diverticulum occurrence, and previous sphincterotomy. Student’s t-test was performed for continuous variables and Chi-square or Fisher’s exact test was performed for categorical variables. Continuous variables were presented as the mean ± standard deviation (SD) for normal distribution and as the median (range) for non-normal distribution. Categorical variables were reported as numbers and percentages. A p value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 23 for Windows software (SPSS, Chicago, Ill., USA).
Results
Patients
We recruited 50 patients (26 males, mean age: 64 ± 17 years) for the DCS group (Fig. 3). By design, patient characteristics were similar between the DCS group and the cERCP group, including gender, age, duodenal diverticulum occurrence, previous biliary sphincterotomy, and CBDS size (Table 1).
Flowchart of patients undergoing DCS-assisted CBDS removal*. CBDS common bile duct stone, DCS digital cholangioscopy. *The reasons for unsuccessful DCS were failed biliary cannulation (n = 1) and missed CBDS clearance by DCS due to dark bile obscuring CBD visibility (n = 1), very small stone fragment (n = 1), and over irrigation causing stone migration to intrahepatic duct (n = 2)
Cannulation
On an intent-to-treat (ITT) basis, successful biliary cannulation was comparable between both groups (98 vs. 98%) (Table 2). In the DCS group, 47 (94%) patients underwent successful biliary cannulation using a standard guidewire-assisted cannulation technique, and 2 (4%) patients underwent a double guidewire technique without fluoroscopy. No cystic duct cannulation was noted. In 1 (2%) patient, non-fluoroscopic bile duct access failed, and a precut sphincterotomy under fluoroscopy was needed to achieve biliary cannulation. In the case-matched cERCP group, 47 (94%) patients underwent successful standard guidewire-assisted cannulation, and 2 (4%) patients underwent a successful double guidewire technique, all under fluoroscopy. In another patient (2%) fluoroscopy-guided biliary cannulation at the first ERCP session failed, and cannulation was ultimately performed using fluoroscopy-guided precut sphincterotomy in a subsequent ERCP.
CBDS clearance
In the DCS group, CBDS clearance was achieved in one round of the DCS procedure in 42 (84%) cases and in two rounds of DCS procedures in 3 (6%) cases as confirmed by occlusion cholangiogram. In 5 (10%) of the cases in the DCS group, conversion to cERCP was needed to complete CBDS clearance due to failure of non-fluoroscopic biliary cannulation (1), dark bile obscuring the visibility of CBD (1), presence of a very small stone fragment (1), and over-irrigation causing the migration of stones into the intrahepatic duct (2) (Fig. 4). In the cERCP group, CBDS clearance was achieved in 49 (98%) of the cases in one ERCP procedure, and in 1 (2%) case, clearance was achieved after two ERCP procedures; the second procedure requiring precut cannulation. Thus, on an ITT basis, CBDS clearance was achieved in one or more rounds of DCS procedures in a single session within the DCS group in 45 (90%) cases, and in one ERCP session in the cERCP group in 49 (98%) cases (p = 0.20) (Table 2). Using the same analysis (ITT), CBDS clearance was achieved in one round of the DCS procedure in a single session in 42 (82%) patients and in one cERCP session in 49 (98%) patients (p = 0.03).
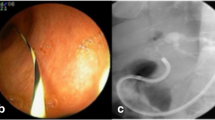
Missed CBDS clearance by DCS due to over irrigation causing stone migration to intrahepatic duct, A initial DCS examination from the hilum to distal CBD confirmed CBDS clearance, B final cholangiogram demonstrated suspicious CBDS at the hilum (black arrow), C subsequent balloon sweep showed a residual CBDS
Duration of the procedure
The overall procedural duration was not significantly different between the DCS group and the cERCP group [DCS (37 ± 10 min) vs. cERCP (34 ± 12 min)]. The mean SpyGlass time for confirmation of CBDS clearance was 5.8 min (range 3–15 min).
Radiation exposure
In the cERCP group, the mean radiation exposure to patients in the prone position was 8415 ± 5502 mGy-cm2, while DCS was performed for successful stone removal without radiation exposure to patients and staff. The mean fluoroscopy time was 257 ± 177 s in the cERCP group. In the DCS group, fluoroscopy was performed at the final cholangiogram as a confirmation for CBDS clearance according to the protocol (DAP 3253 ± 3536 mGy-cm2).
Adverse events
There were no differences in adverse events related to the procedures between the DCS group and the cERCP group (10 vs. 16%), including mild pancreatitis (4 vs. 4%), post-sphincterotomy bleeding requiring adrenaline injection (4 vs. 8%), type II duodenal perforation managed conservatively without surgery (0 vs. 2%) and cholangitis (2 vs. 2%). All patients with prior cholangitis improved without worsening of their condition. No death was reported. The median length of hospital stay was comparable in both groups.
Discussion
Previous studies showed that radiation-free ERCP guided by EUS or IDUS could be used for confirmation of CBDS clearance; however, the data were limited by the study design heterogeneity and lack of control group [9,10,11]. With EUS guidance, the endoscopist needs to exchange the duodenoscope to the EUS scope to confirm CBDS clearance, whereas the IDUS catheter probe can be advanced through the duodenoscope for scanning. In a study from Korea on IDUS-directed management of CBDS without radiocontrast cholangiography [10], IDUS was applied in 38 patients to verify CBDS clearance after saline solution irrigation. Following endoscopic sphincterotomy, IDUS-directed CBDS removal was successfully performed without radiocontrast cholangiography in 26 patients during the first session. In the remaining nine patients, a second IDUS to confirm CBDS clearance was required. However, a final cholangiogram was not performed as the gold standard in that study. The authors mentioned the limitations of IDUS, including the inability to provide a clear view of the bile duct lumen because of air bubbles.
Recently, a prospective randomized trial from Hat Yai, Thailand demonstrated that the CBDS clearance rate of EUS-assisted ERCP was inferior to the conventional ERCP group (85 vs. 100%) (p = 0.002) [16]. They counted the CBDS number under EUS and tried to match the number when they retrieved the stones. Of note, a final cholangiogram was still required to confirm CBDS clearance in the EUS-assisted group. Although our study performed the final cholangiogram as the gold standard for CBDS clearance, the actual technique that we recommended does not require cholangiogram as we determined that cholangioscopy alone was enough. In our study, since residual stones were seen during cholangioscopy in 3 (6%) patients, then the second round of non-fluoroscopic balloon sweep was attempted until cholangioscopy confirmed CBD clearance. Unlike direct cholangioscopy, only counting the CBDS number under EUS may not precisely match the real CBDS number. In our opinion, under EUS, it is quite difficult to match the CBDS by its size and shape to the actual endoscopic finding. Moreover, some of CBDSs may become fragmented during the stone retrieval procedure.
A recent study by Barakat et al. showed successful fluoroscopy-free biliary cannulation and stone extraction under cholangioscopy guidance performed by a single endoscopist in all 40 patients with non-complex CBDS. However, the control group (cERCP) was lacking [17]. Based on the Barakat et al. protocol, cholangioscopy was applied before CBDS removal for documentation of the number and location of CBDS, and again after CBDS removal for confirmation of CBD clearance. Our study is the first study that compared the feasibility and safety of radiation-free DCS (n = 50) with cERCP (n = 50) for CBDS clearance using a propensity score matching design. According to our protocol, all procedures were performed by one of the four experienced endoscopists, and cholangioscopy was done only after stone removal to confirm CBDS clearance and to reduce the risk of bacteremia, since we evaluated the number and location of CBDS by pre-procedure imaging studies. The success rate of DCS for CBDS clearance without conversion to ERCP was 90% and trended to lower than cERCP success rates (100%) (without statistical significance). Nevertheless, DCS offers secondary benefits including being a radiation-free alternative, and it also allows performance of CBDS clearance without the need for fluoroscopic equipment. Furthermore, no serious adverse events were observed in the DCS group. Although we expected the potential risk of cholangitis with direct cholangioscopy to be significantly based on previous studies using an ultraslim endoscope (14%) [18,19,20], in our series, we experienced only 2% of new cholangitis cases using DCS. This very low rate of cholangitis may be explained by the dedicated irrigation and aspiration channels of DCS that reduced the water-pressure load.
Compared to the previous series, our data showed a higher success rate of non-fluoroscopic biliary cannulation (98%) [10, 11, 16]. In our series, the success rate of biliary cannulation without the use of fluoroscopy was comparable to that with fluoroscopy assistance (98 vs. 98%). Moreover, the double guidewire technique could be performed without fluoroscopy in two patients. These data suggest that the double guidewire technique without fluoroscopy is technically feasible for DCS. Nevertheless, advanced techniques for bile duct cannulation, including precut under fluoroscopy, may be eventually required, as we performed for one of our patients.
In our series, residual small CBDS were missed by DCS and were detected in the final cholangiogram with a balloon sweep in four patients (8%). The reasons for missed CBDS clearance by DCS included dark bile obscuring CBD visibility, a very small stone fragment size of 3 mm, and over irrigation causing stone migration into the left intrahepatic duct (Fig. 4). Our data suggest that these conditions deserve special attention since they could limit the effectiveness of DCS. Notably, in a case with very dark bile (one case in this series) or turbid bile, more time may be needed to carefully aspirate and irrigate the ducts to optimize visualization. In addition, carefully aspirating the ducts before irrigating and balancing amounts of bile aspiration and irrigation water while iteratively checking the ductal tree as best as possible to minimize the risk of stone migration into deep hepatic ducts (two cases in this series) is strongly recommended.
Our data showed similar total procedure time between the DCS group and cERCP group. Of note, our endoscopists had performed more than 20 Legacy cholangioscopies under fluoroscopy prior to this study. The mean SpyGlass procedure time was 6 min. During the learning curve (n = 42) using an ultraslim cholangioscopic system, significant variability in the results was reported in the first nine procedures, followed by a steady improvement in the results of subsequent cholangioscopic procedures [21]. However, no data on the learning curves for digital SpyGlass procedures have been published. We foresee that the learning curve for DCS would be faster than that previously published on the Legacy system.
Although radiation risks associated with ERCP for endoscopists are relatively low, the risk of radiation-induced cancer was 10% for a lifetime exposure of 1 Sieverts (Sv) [22, 23]. We demonstrated DCS as a radiation-free alternative technique for uncomplicated CBDS removal. In certain patients, such as those that are pregnant, or immobilized critically ill patients, DCS may be the only option to remove CBDS as it can be performed at bedside.
Our study is limited by the retrospective nature of the cERCP group. However, selection bias and confounding factors of our data are reduced by the propensity score matching analysis. Since the data were limited to a CBDS size < 15 mm, we found that DCS is easy to perform and provides a comparable duration of procedure for patients with small CBDS when compared to cERCP. However, in clinical practice, the previous imaging studies may not correctly help for selection of ideal candidate for DCS. Although there was very low rate of cholangitis in the DCS group, the volume of saline irrigation during cholangioscopy was not mentioned in our study. More importantly, it should be realized that pregnant patients and critically ill immobilized patients, who were excluded in this study, cannot be put in prone position, and supine ERCP is required. This in turn made it difficult for us to recruit such cases as we had to enroll the regular CBDS cases and extrapolated their results to those particular cases. Furthermore, cholangioscopy can be more challenging in patients in the supine position due to the degree of technical difficulty. Although cholangitis patients could potentially benefit from radiation-free DCS for CBDS removal, DCS in such patients should be done with care as it is generally not advisable to perform cholangioscopy in patients with severe cholangitis. Another limitation is that all procedures were performed by experienced endoscopists. We need more data on the DCS learning curve for community practitioners. Last, we did not perform a cost-effectiveness analysis on DCS as the technique requires a single-use device and since the cost that incurs from a single-use SpyScope is much more expensive than the routine ERCP cost for CBD clearance under standard fluoroscopy.
In conclusion, our data confirmed the feasibility of DCS for CBDS clearance as it showed efficacy and safety comparable to those of cERCP for uncomplicated CBDS. DCS offers the ability to perform CBDS clearance without the need for fluoroscopy unit and can avoid radiation exposure while ERCP under fluoroscopy remains the current standard of care in patients with CBDS. Particular conditions could limit the effectiveness of DCS; however, these limitations may be overcome in the future by procedural optimization.
Abbreviations
- ERCP:
-
Endoscopic retrograde cholangiopancreatography
- CBDS:
-
Common bile duct stone
- EUS:
-
Endoscopic ultrasound
- IDUS:
-
Intraductal ultrasound
- DCS:
-
Digital cholangioscopy
- cERCP:
-
conventional ERCP
- DAP:
-
Dose area product
- TUS:
-
Transabdominal ultrasonography
- CT:
-
Computed tomography
- MRCP:
-
Magnetic resonance cholangiopancreatography
- SD:
-
Standard deviation
- ASGE:
-
American Society for Gastrointestinal Endoscopy
References
Campbell N, Sparrow K, Fortier M, Ponich T (2002) Practical radiation safety and protection for the endoscopist during ERCP. Gastrointest Endosc 55:552–557
Committee AT, Pedrosa MC, Farraye FA, Shergill AK, Banerjee S, Desilets D, Diehl DL, Kaul V, Kwon RS, Mamula P, Rodriguez SA, Varadarajulu S, Song LM, Tierney WM (2010) Minimizing occupational hazards in endoscopy: personal protective equipment, radiation safety, and ergonomics. Gastrointest Endosc 72:227–235
Stavropoulos S, Larghi A, Verna E, Stevens P (2005) Therapeutic endoscopic retrograde cholangiopancreatography without fluoroscopy in four critically ill patients using wire-guided intraductal ultrasound. Endoscopy 37:389–392
Wu W, Faigel DO, Sun G, Yang Y (2014) Non-radiation endoscopic retrograde cholangiopancreatography in the management of choledocholithiasis during pregnancy. Dig Endosc 26:691–700
Shelton J, Linder JD, Rivera-Alsina ME, Tarnasky PR (2008) Commitment, confirmation, and clearance: new techniques for nonradiation ERCP during pregnancy (with videos). Gastrointest Endosc 67:364–368
Sharma SS, Maharshi S (2008) Two stage endoscopic approach for management of choledocholithiasis during pregnancy. J Gastrointest Liver Dis 17:183–185
Ersoz G, Turan I, Tekin F, Ozutemiz O, Tekesin O (2016) Nonradiation ERCP with endoscopic biliary sphincterotomy plus papillary balloon dilation for the treatment of choledocholithiasis during pregnancy. Surg Endosc 30:222–228
Trindade AJ, Brun A, Vamadevan AS, Sideridis K, Sejpal DV, Mayo PH, Khanijo S, Koenig SJ (2015) Use of bedside transabdominal US in facilitating emergent intensive care unit ERCP without fluoroscopy. Gastrointest Endosc 81:1268–1269
Artifon EL, Kumar A, Eloubeidi MA, Chu A, Halwan B, Sakai P, Bhutani MS (2009) Prospective randomized trial of EUS versus ERCP-guided common bile duct stone removal: an interim report (with video). Gastrointest Endosc 69:238–243
Park SY, Park CH, Lim SU, Cho EA, Lee DH, Jun CH, Kim HS, Choi SK, Rew JS (2015) Intraductal US-directed management of bile duct stones without radiocontrast cholangiography. Gastrointest Endosc 82:939–943
Shah JN, Bhat YM, Hamerski CM, Kane SD, Binmoeller KF (2016) Feasibility of nonradiation EUS-based ERCP in patients with uncomplicated choledocholithiasis (with video). Gastrointest Endosc 84:764–769
Palazzo L, Girollet PP, Salmeron M, Silvain C, Roseau G, Canard JM, Chaussade S, Couturier D, Paolaggi JA (1995) Value of endoscopic ultrasonography in the diagnosis of common bile duct stones: comparison with surgical exploration and ERCP. Gastrointest Endosc 42:225–231
Ishida Y, Itoi T, Okabe Y (2016) Types of peroral cholangioscopy: how to choose the most suitable type of cholangioscopy. Curr Treat Options Gastroenterol 14:210–219
Committee ASoP, Maple JT, Ikenberry SO, Anderson MA, Appalaneni V, Decker GA, Early D, Evans JA, Fanelli RD, Fisher D, Fisher L, Fukami N, Hwang JH, Jain R, Jue T, Khan K, Krinsky ML, Malpas P, Ben-Menachem T, Sharaf RN, Dominitz JA (2011) The role of endoscopy in the management of choledocholithiasis. Gastrointest Endosc 74:731–744
Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N (1991) Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc 37:383–393
Netinatsunton N, Sottisuporn J, Attasaranya S, Witeerungrot T, Siripun A, Pattarapuntakul T, Ovartlarnporn B (2017) Prospective randomized trial of EUS-assisted ERCP without fluoroscopy versus ERCP in common bile duct stone. Gastrointest Endosc. https://doi.org/10.1016/j.gie.2017.03.1539
Barakat MT, Girotra M, Choudhary A, Huang RJ, Sethi S, Banerjee S (2017) A prospective evaluation of radiation-free direct solitary cholangioscopy for the management of choledocholithiasis. Gastrointest Endosc. https://doi.org/10.1016/j.gie.2017.07.042
Chathadi KV, Chen YK (2009) New kid on the block: development of a partially disposable system for cholangioscopy. Gastrointest Endosc Clin N Am 19:545–555
Moon JH, Choi HJ (2013) The role of direct peroral cholangioscopy using an ultraslim endoscope for biliary lesions: indications, limitations, and complications. Clin Endosc 46:537–539
Sethi A, Chen YK, Austin GL, Brown WR, Brauer BC, Fukami NN, Khan AH, Shah RJ (2011) ERCP with cholangiopancreatoscopy may be associated with higher rates of complications than ERCP alone: a single-center experience. Gastrointest Endosc 73:251–256
Weigt J, Kandulski A, Malfertheiner P (2015) Technical improvement using ultra-slim gastroscopes for direct peroral cholangioscopy: analysis of the initial learning phase. J Hepatobiliary Pancreat Sci 22:74–78
Raijman I (2016) Performing endoscopic retrograde cholangiography without radiation exposure: are we ready for it? Gastrointest Endosc 84:770–772
Hendee WR (1992) Estimation of radiation risks. BEIR V and its significance for medicine. JAMA 268:620–624
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Wiriyaporn Ridtitid, Thanawat Luangsukrerk, Phonthep Angsuwatcharakon, Panida Piyachaturawat, Prapimphan Aumpansub, Cameron Hurst, Roongruedee Chaiteerakij, Pradermchai Kongkam, and Rungsun Rerknimitr have no conflict of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Ridtitid, W., Luangsukrerk, T., Angsuwatcharakon, P. et al. Uncomplicated common bile duct stone removal guided by cholangioscopy versus conventional endoscopic retrograde cholangiopancreatography. Surg Endosc 32, 2704–2712 (2018). https://doi.org/10.1007/s00464-017-5966-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5966-2