Abstract
Background
Laparoscopic surgery is associated with a high incidence of postoperative nausea and vomiting (PONV). The use of CO2 pneumoperitoneum has been proposed as a potential cause of high PONV incidence. However, intraoperative hypercarbia may be related to enhanced perfusion to the main effector sites for PONV, including the brain and gastrointestinal tract. In this study, we investigated whether an increase in intraoperative CO2 partial pressure in arterial blood (PaCO2) reduces the incidence of PONV.
Methods
This study enrolled 400 female patients aged 20–60 years who were undergoing laparoscopic gynecologic surgery. The patients were allocated randomly to one of three groups with the following intraoperative PaCO2 levels: 36–40 mmHg (Group 1), 41–45 mmHg (Group 2), or 46–50 mmHg (Group 3). The anesthetic regimen used a standardized total intravenous anesthesia consisting of propofol and remifentanil for all patients. The arterial blood gas analysis was performed to identify the difference in CO2 partial pressure between arterial blood and end-tidal gas. The PONV incidence was evaluated for the periods of 0–2, 2–6, and 6–24 h after anesthesia. The incidence and severity of PONV and the administration of rescue antiemetics were recorded.
Results
The three groups were comparable for the patient, anesthesia, and surgical characteristics. The average PaCO2 level during surgery was 38–39, 43–44, and 47–48 mmHg in Groups 1, 2, and 3, respectively. The incidence and severity of PONV and use of rescue antiemetics were not significantly different among the groups. The overall incidence of nausea during the first 24-h postoperative period was 54, 48, and 50% in Groups 1, 2, and 3, respectively (P = 0.593).
Conclusion
Our data suggest that mild to moderate intraoperative hypercapnia did not decrease the incidence and severity of PONV or the requirement for rescue antiemetics after gynecologic laparoscopic surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Postoperative nausea and vomiting (PONV) is the most common adverse effect following general anesthesia and surgery. Although the etiology of PONV remains unclear, the contributing factors include the following: gender, smoking, a history of motion sickness or previous PONV, anesthetic technique, type of surgery, and use of opioids. Laparoscopic surgery reduces postoperative pain and hospital stay and can lead to a faster return to normal activities than laparotomic surgery [1, 2]. However, there is a higher incidence of PONV associated with laparoscopic surgery [3]. Although compression of the gastrointestinal mucosa by the pneumoperitoneum could induce intestinal ischemia and serotonin release that may lead to PONV [4], the cause of the high incidence of PONV after laparoscopic surgery remains unknown.
The use of CO2 pneumoperitoneum with 10–15 mmHg of intra-abdominal pressure (IAP) is the most common technique used to create an adequate working space in laparoscopy. In addition to the increased IAP caused by CO2 pneumoperitoneum, the Trendelenburg position results in elevation of the diaphragm and the abdominal contents into the chest during gynecologic and colorectal laparoscopic surgery. As a result, air-way pressure is increased and pulmonary compliance is decreased. These changes could cause hypoventilation and dead-space ventilation [5]. Hypoventilation increases the CO2 partial pressure in the arterial blood (PaCO2), and dead-space ventilation increases the difference between PaCO2 and end-tidal CO2 (ETCO2). Furthermore, CO2 gas is absorbed across the peritoneum and results in hypercarbia during CO2 pneumoperitoneum [6]. The combined effects of CO2 absorption and hypoventilation significantly increase PaCO2. The PaCO2 is indirectly monitored by ETCO2 during general anesthesia, and the increased difference between PaCO2 and ETCO2 can make it difficult to estimate PaCO2 by ETCO2. Therefore, the PaCO2 may be underestimated and could lead to hypercarbia during laparoscopic gynecologic surgery.
CO2 is a potent vasodilator, and intraoperative hypercarbia may be related to enhanced perfusion to the main effector sites for PONV, including the brain and gastrointestinal tract. Therefore, we hypothesized the PaCO2 levels affect the incidence of PONV following laparoscopic surgery. We conducted a prospective randomized double-blinded study to investigate the hypothesis that an increase in the level of intraoperative PaCO2 reduces the incidence of PONV after laparoscopic gynecologic surgery.
Materials and methods
This prospective randomized double-blinded study was approved by the institutional review board of Chonbuk National University Hospital and was registered with the WHO International Clinical Trials Registry Platform (KCT0001125). We obtained written informed consent and enrolled 400 female patients (aged 20–60 years with American Society of Anesthesiologists physical status I or II, and undergoing laparoscopic gynecologic surgery) in the study. The patient exclusion criteria included the following: history of motion sickness or PONV, diabetes mellitus, chronic obstructive pulmonary disease, gastrointestinal disease, smokers, actively menstruating, body mass index of <16 or >35, and use of antiemetics or steroids within 72 h prior to surgery. The four major risk factors of PONV proposed by Apfel et al. [7] are female gender, non-smoker, the use of postoperative opioids, and a history of motion sickness or PONV. The basic inclusion criteria of this study were female gender, non-smoker, and anticipated use of opioids postoperatively. Therefore, patients with three of the four major risk factors of PONV were selected. We used computer-generated random numbers to allocate patients to one of the following three intraoperative PaCO2-level groups: 36–40 mmHg (Group 1), 41–45 mmHg (Group 2), or 46–50 mmHg (Group 3).
The anesthetic regimen was standardized with total intravenous anesthesia for all patients. No patient received pre-anesthetic medication. All patients were monitored by ECG, noninvasive blood pressure, temperature, pulse oximetry, and capnography. The anesthesia was induced with 3–5 μg/ml of the effect-site concentration of propofol (Fresofol MCT® 2%, Fresenius Kabi, Graz, Austria) and 4 ng/ml of the effect-site concentration of remifentanil (Ultiva®, GlaxoSmithKline, Parma, Italy). The propofol and remifentanil were infused using an effect-site target-controlled infusion pump (Orchestra® Base Primea, Fresenius Vial, Brézins, France) with the Marsh and Minto models, respectively. After the patients lost consciousness, we administered 1.0 mg/kg of rocuronium and orotracheal intubation was performed. All patients received 5 mg of dexamethasone for prophylaxis of PONV at anesthesia induction. The controlled mechanical ventilation was maintained with a TV of 6–8 ml/kg of ideal body weight and an inspiratory to expiratory ratio of 1:2 with 5 cm H2O of PEEP. The arterial blood gas analysis was performed to identify the difference in CO2 partial pressure between PaCO2 and ETCO2 at 5 min after endotracheal intubation and 5 min after CO2 pneumoperitoneum. All patients underwent laparoscopic gynecologic surgery under 12 mmHg of CO2 pneumoperitoneum. The PaCO2 was measured indirectly by corrected-ETCO2 (the ETCO2 plus the difference between PaCO2 and ETCO2) during surgery. The corrected-ETCO2 values were recorded every 10 min during surgery. The ventilation frequency was adjusted to maintain the predetermined PaCO2 range for the group, and the values were maintained until the end of surgery. The arterial blood pressure was maintained within 20% of the pre-anesthetic values. The effect-site concentrations of propofol and remifentanil were adjusted according to blood pressure. A balanced salt solution was infused at a rate of 6 ml/kg/h during surgery.
The patients were administered 2 μg/kg of fentanyl upon closure of the skin. All patients were equipped with a patient-controlled analgesia (PCA) device (Accumate 1100®, Wooyoung Meditech, Seoul, Korea). The 60 ml analgesic solution contained 20 μg/kg of fentanyl in normal saline. The PCA settings included 0.5 ml/h background infusion and a demand bolus dose of 1.0 ml. The lockout interval was 8 min, and the patients were limited to 6 uses per hour. The propofol and remifentanil infusions were stopped at the completion of surgery. The residual neuromuscular blockade was antagonized with neostigmine and glycopyrrolate before the trachea was extubated.
The patients were monitored for 2 h in the post-anesthesia care unit (PACU) and interviewed in the ward postoperatively at 6 and 24 h to assess nausea and vomiting. PONV during periods of 0–2, 2–6, and 6–24 h after anesthesia was evaluated by an anesthesiologist blinded to the study groups or by the spontaneous complaints of the patients. Nausea was defined as a subjectively unpleasant sensation associated with awareness of the urge to vomit. Vomiting was defined as the forceful expulsion of gastric contents from the mouth. The patients were provided rescue antiemetics immediately at the discretion of the attending anesthesiologists, in response to nausea and vomiting, or at the patient’s request. The attending anesthesiologists were unaware of the group identities. The first-line rescue antiemetic was 0.75 mg of palonosetron, and 10 mg of metoclopramide was the second-line antiemetic.
Any episode of nausea or vomiting during the 24-h period following surgery was considered a PONV occurrence. The incidence and severity of PONV and administration of rescue antiemetics were recorded. The nausea severity was recorded using a visual analog scale (nausea-VAS; where 0 cm = no nausea and 10 cm = worst possible nausea) at postoperative 2, 6, and 24 h or when the patient complained of nausea or vomiting. The level of postoperative pain was evaluated using a VAS (pain-VAS; where 0 cm = no pain and 10 cm = worst possible pain) simultaneously with the PONV evaluation.
Statistical analysis
The sample size was predetermined by proportions sample size using SigmaPlot 12.0 (Systat Software Inc., San Jose, USA) based on the assumption that the incidence of PONV, which was regarded as the primary endpoint, would be 60% in Group 1 and 40% in Group 3. A sample of 107 patients was required in each group with a significance level of 0.05 (α = 0.05) and a power of 0.8 (β = 0.20). The total sample size was enlarged to 400 patients to allow for attrition.
All statistical analyses were performed with SigmaPlot 12.0. The continuous variables were analyzed with a one-way analysis of variance. The incidence of PONV and use of rescue antiemetics were analyzed using the Chi-square test. The Holm–Sidak method and Bonferroni correction were used for multiple comparisons after the ANOVA and Chi-square test, respectively. All data are expressed as the mean ± standard deviation or patient number and percentage. A P value <0.05 was considered statistically significant.
Results
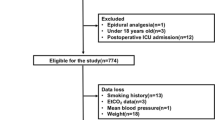
There were 381 patients who completed the study, and the subject flow diagram is shown in Fig. 1. The three groups were similar with respect to patient characteristics, anesthesia, pneumoperitoneum time, volume of fluids administered, and surgery types (Table 1). The average PaCO2 level during surgery was 38–39, 43–44, and 47–48 mmHg in Group 1, 2, and 3, respectively (Fig. 2). The difference between PaCO2 and ETCO2 at 5 min after tracheal intubation was 2.8 ± 1.7 mmHg in Group 1, 2.7 ± 2.1 mmHg in Group 2, and 2.6 ± 1.9 mmHg in Group 3 (P = 0.654). The difference increased after pneumoperitoneum to 3.7 ± 2.3, 3.6 ± 2.3, and 3.6 ± 2.3 mmHg in Groups 1, 2, and 3, respectively.
Intraoperative partial pressure of carbon dioxide in the arterial blood (PaCO2) was significantly higher in Groups 2 and 3 compared with Group 1. IT-5: 5 min after intubation; PP-5, PP-10, PP-20, and PP-40: 5, 10, 20, and 40 min after pneumoperitoneum, respectively; PP-End: at the end of pneumoperitoneum; SC: at skin closure
The incidence of PONV and use of rescue antiemetics were not significantly different among the three groups (Table 2). The overall incidences of nausea during the first postoperative 24 h were 54, 48, and 50% in Groups 1, 2, and 3, respectively (P = 0.593). The intensity of postoperative nausea evaluated with the VAS was similar among the three groups for patients who had PONV (Table 3). The dose of fentanyl given during the postoperative 24 h was comparable among the three groups (Group 1; 673 ± 228 μg, Group 2; 667 ± 240 μg, Group 3; 645 ± 204 μg, P = 0.584). There were no differences in pain-VAS among the three groups in each time period (Table 4).
Discussion
The use of CO2 pneumoperitoneum has been proposed as a potential cause of high PONV incidence [8, 9]. During laparoscopic surgery, the CO2 pneumoperitoneum results in two physiological derangements compared with laparotomic surgery. These changes include increased IAP and hypercapnia. The increased IAP could decrease intestinal blood flow [4], and because the intestinal mucosa is highly metabolic and poorly tolerates ischemia [10], even brief periods of ischemia can trigger the release of neurotransmitters, such as serotonin, that could lead to PONV [11]. Moreover, increased IAP may increase intracranial pressure (ICP) [12], and elevated ICP is a cause of nausea and vomiting [13]. However, a previous study reported that low-pressure CO2 pneumoperitoneum (8 mmHg) does not reduce the incidence and severity of PONV compared with 13 mmHg of IAP [14]. Although the CO2 pneumoperitoneum group had a significantly higher incidence of PONV than the abdominal wall-lifting group in a recent meta-analysis [15], the abdominal wall-lifting method did not use CO2 insufflation or increase IAP.
CO2 has a vasodilating effect, and hypercapnia improves global hemodynamics, tissue perfusion, and oxygenation during general anesthesia [16–18]. A retrospective study reported there is a significant association between higher intraoperative ETCO2 and shorter length of hospital stay after colon resection and hysterectomy [19]. The increased tissue perfusion and oxygenation caused by hypercapnia may contribute to improved postoperative condition and a shorter hospital stay. PONV is also a common factor that can increase hospital stay and morbidity after surgery. Intestinal ischemia is proposed as an important mechanism of PONV [4, 20], and we hypothesized that decreased intestinal mucosal perfusion can be offset by the vasodilating effect of hypercapnia. Thus, hypercapnia may reduce the incidence of PONV after laparoscopic gynecologic surgery. However, we did not find any differences in the incidence and severity of PONV in patients with mild to moderate hypercapnia compared with those with normocapnia. CO2 has dilatory effects on cerebral vessels and may increase ICP. Nevertheless, the favorable effect of hypercapnia on tissue perfusion and oxygenation may counteract the emetic effect of increased ICP.
Two recent studies investigated the effects of ETCO2 on the incidence of PONV [21, 22]. However, the study results were contradictory. In patients who underwent percutaneous nephrolithotomy (PCNL), mild intraoperative hypercapnia (ETCO2 43–45 mmHg) resulted in a lower incidence and severity of PONV compared with normocapnia and hypocapnia [21]. However, PCNL was performed in the prone position without CO2 pneumoperitoneum. Therefore, IAP was not increased, and the capillary blood flow of the intestinal mucosa may not have been obstructed during surgery. Conversely, the other study demonstrated intraoperative ETCO2 and venous CO2 levels are independent risk factors for postoperative vomiting in pediatric patients [22]. The incidence of vomiting is only evaluated for PONV investigation because evaluation of nausea is difficult in pediatric patient. However, the mechanisms of nausea and vomiting are not identical. The sample sizes in both studies were too small to investigate the incidence of PONV. Thus, a large randomized controlled study is needed to evaluate the effects of hypercapnia on PONV. In general, a large-scale study involving more than 100 patients per group is recommended for PONV investigations [23].
In the current study, we reported that 65% of patients in the control group (Group 1) had PONV during the postoperative 24-h period. This incidence was slightly lower than previous studies in patients who underwent laparoscopic gynecologic surgery (69–70%) [24, 25]. The difference could be due to the use of prophylactic antiemetic medication (dexamethasone) and the anesthetic technique (total intravenous anesthesia with propofol versus inhalational anesthesia with N2O). In addition to the four major risk factors, the other contributing factors of PONV, including operation type and time and anesthetic technique, were well controlled in this study. Therefore, the outcomes of the current study could be attributed to differences in PaCO2.
There are two limitations in this study. First, the effects of hypocapnia on PONV were not evaluated. Because the CO2 levels of the most patients were normocapnia or hypercapnia during laparoscopic surgery, the hypocapnia group was excluded in the current study. However, the effects of hypocapnia should be examined in further studies to evaluate the effects of PaCO2 on PONV. Second, the PaCO2 or ETCO2 values were not monitored in the PACU. The patients breathed spontaneously, and they may be hypoventilated and hypercapnic at the early period in the PACU. We could not exclude the effects of hypercapnia on the normocapnia group if the patients were hypoventilated.
In conclusion, mild to moderate intraoperative hypercapnia did not decrease the incidence and severity of PONV or the requirement of rescue antiemetics compared with normocapnia after gynecologic laparoscopic surgery.
References
McMahon AJ, Russell IT, Baxter JN, Ross S, Anderson JR, Morran CG, Sunderland G, Galloway D, Ramsay G, O’Dwyer PJ (1994) Laparoscopic versus minilaparotomy cholecystectomy: a randomised trial. Lancet 343:135–138
Chen HH, Wexner SD, Iroatulam AJ, Pikarsky AJ, Alabaz O, Nogueras JJ, Nessim A, Weiss EG (2000) Laparoscopic colectomy compares favorably with colectomy by laparotomy for reduction of postoperative ileus. Dis Colon Rectum 43:61–65
Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, Meyer TA, Watcha M, Chung F, Angus S, Apfel CC, Bergese SD, Candiotti KA, Chan MT, Davis PJ, Hooper VD, Lagoo-Deenadayalan S, Myles P, Nezat G, Philip BK, Tramer MR, Society for Ambulatory A (2014) Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg 118:85–113
Goll V, Akca O, Greif R, Freitag H, Arkilic CF, Scheck T, Zoeggeler A, Kurz A, Krieger G, Lenhardt R, Sessler DI (2001) Ondansetron is no more effective than supplemental intraoperative oxygen for prevention of postoperative nausea and vomiting. Anesth Analg 92:112–117
Koivusalo AM, Kellokumpu I, Scheinin M, Tikkanen I, Makisalo H, Lindgren L (1998) A comparison of gasless mechanical and conventional carbon dioxide pneumoperitoneum methods for laparoscopic cholecystectomy. Anesth Analg 86:153–158
Ho HS, Saunders CJ, Gunther RA, Wolfe BM (1995) Effector of hemodynamics during laparoscopy: CO2 absorption or intra-abdominal pressure? J Surg Res 59:497–503
Apfel CC, Laara E, Koivuranta M, Greim CA, Roewer N (1999) A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology 91:693–700
Watcha MF, White PF (1992) Postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthesiology 77:162–184
Fredman B, Jedeikin R, Olsfanger D, Flor P, Gruzman A (1994) Residual pneumoperitoneum: a cause of postoperative pain after laparoscopic cholecystectomy. Anesth Analg 79:152–154
Beuk RJ, Heineman E, Tangelder GJ, Kurvers HA, Bonke HJ, Oude Egbrink MG (1997) Effects of different durations of total warm ischemia of the gut on rat mesenteric microcirculation. J Surg Res 73:14–23
Marston A (1977) Responses of the splanchnic circulation to ischaemia. J Clin Pathol Suppl (R Coll Pathol) 11:59–67
Josephs LG, Este-McDonald JR, Birkett DH, Hirsch EF (1994) Diagnostic laparoscopy increases intracranial pressure. J Trauma 36:815–819
Andrews PL (1992) Physiology of nausea and vomiting. Br J Anaesth 69:2S–19S
Kim D-K, Cheong I-Y, Lee G-Y, Cho J-H (2006) Low Pressure (8 mmHg) Pneumoperitoneum does not Reduce the Incidence and Severity of Postoperative Nausea and Vomiting (PONV) following Gynecologic Laparoscopy. Korean J Anesthesiol 50:S36–S42
Ren H, Tong Y, Ding XB, Wang X, Jin SQ, Niu XY, Zhao X, Li Q (2014) Abdominal wall-lifting versus CO2 pneumoperitoneum in laparoscopy: a review and meta-analysis. Int J Clin Exp Med 7:1558–1568
Mas A, Saura P, Joseph D, Blanch L, Baigorri F, Artigas A, Fernandez R (2000) Effect of acute moderate changes in PaCO2 on global hemodynamics and gastric perfusion. Crit Care Med 28:360–365
Akca O, Doufas AG, Morioka N, Iscoe S, Fisher J, Sessler DI (2002) Hypercapnia improves tissue oxygenation. Anesthesiology 97:801–806
Fleischmann E, Herbst F, Kugener A, Kabon B, Niedermayr M, Sessler DI, Kurz A (2006) Mild hypercapnia increases subcutaneous and colonic oxygen tension in patients given 80% inspired oxygen during abdominal surgery. Anesthesiology 104:944–949
Wax DB, Lin HM, Hossain S, Porter SB (2010) Intraoperative carbon dioxide management and outcomes. Eur J Anaesthesiol 27:819–823
Pusch F, Berger A, Wildling E, Zimpfer M, Moser M, Sam C, Krafft P (2002) Preoperative orthostatic dysfunction is associated with an increased incidence of postoperative nausea and vomiting. Anesthesiology 96:1381–1385
Saghaei M, Matin G, Golparvar M (2014) Effects of intra-operative end-tidal carbon dioxide levels on the rates of post-operative complications in adults undergoing general anesthesia for percutaneous nephrolithotomy: a clinical trial. Adv Biomed Res 3:84
Altay N, Yalcin S, Aydogan H, Kucuk A, Yuce HH (2015) Effects of end tidal CO2 and venous CO2 levels on postoperative nausea and vomiting in paediatric patients. Eur Rev Med Pharmacol Sci 19:4254–4260
Gan TJ, Meyer T, Apfel CC, Chung F, Davis PJ, Eubanks S, Kovac A, Philip BK, Sessler DI, Temo J, Tramer MR, Watcha M (2003) Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg 97:62–71
Graczyk SG, McKenzie R, Kallar S, Hickok CB, Melson T, Morrill B, Hahne WF, Brown RA (1997) Intravenous dolasetron for the prevention of postoperative nausea and vomiting after outpatient laparoscopic gynecologic surgery. Anesth Analg 84:325–330
Boehler M, Mitterschiffthaler G, Schlager A (2002) Korean hand acupressure reduces postoperative nausea and vomiting after gynecological laparoscopic surgery. Anesth Analg 94:872–875
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Ji-Seon Son, Ji-Youn Oh, and Seonghoon Ko have no conflict of interests or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Son, JS., Oh, JY. & Ko, S. Effects of hypercapnia on postoperative nausea and vomiting after laparoscopic surgery: a double-blind randomized controlled study. Surg Endosc 31, 4576–4582 (2017). https://doi.org/10.1007/s00464-017-5519-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5519-8