Abstract
Background
Laparoscopic adjustable gastric banding (LAGB) was a popular procedure in the USA and Europe in the past decade. However, its use has currently declined. Band erosion (BE) is a rare complication after LAGB with a reported incidence rate of 1.46 %. Controversies exist regarding the management, approach and timing for the band removal. The aim of this study is to describe the rate, clinical presentation and perioperative outcomes of BEs at our institution and provide overall recommendations regarding the diagnosis and management of BE.
Materials and methods
This study is a single-center, retrospective review of a prospectively maintained database. Data were collected from all consecutive patients who underwent a LAGB and band revisional surgeries at the University of Illinois Hospital and Health Sciences System from December 2008 to September 2015. We identified patients who underwent gastric band removal due to a BE and analyzed their outcomes.
Results
A total of 576 LAGBs were performed at our institution. Nine patients underwent surgery for BE at our hospital. The average time between the primary surgery and the removal of the band was 68.5 (42.9) months. Abdominal pain, nausea and/or vomiting were the most frequently mentioned symptoms. In all patients, a minimally invasive approach was used to remove the band. The mean length of hospitalization was 2.6 (1.1) days. The only complication was a pneumonia (n = 1).
Conclusions
BE is one of the most severe complications of LAGB. The minimally invasive approach provided us with the opportunity to repair the fistula, and it was associated with a prompt recovery with very little morbidity. In general, it is recommended that the band be removed at the time of the diagnosis of the BE. Endoscopic band removal can be utilized with patients who have a more advanced BE and migration into the gastric lumen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In the USA, 35.7 % of the adult population is obese (BMI ≥ 30) [1], and 6.6 % of the USA adult population is morbidly (or severely) obese (BMI ≥ 40) [2]. Bariatric surgery has been shown to be one of the most effective treatments for obesity [3, 4] and for the improvement of medical comorbidities [4]. During 2014, 193,000 bariatric surgeries were performed in the USA in accredited centers [5], but still this represents only 1 % of the individuals that may be eligible for bariatric surgery [6]. According to guidelines established by the National Institute of Heart, Lung and Blood Expert Panel on the Identification, Evaluation, and Treatment of Obesity in Adults [7], bariatric surgery is appropriate for those with a BMI > 40 or BMI > 35 and 1 or more significant comorbidities, when less invasive methods of weight loss have failed and the patient is at risk for obesity-associated morbidity and mortality [7, 8].
Laparoscopic adjustable gastric banding (LAGB) was one of the most popular procedures in the USA and Europe in the past decade [9, 10]. However, compared to 2011, when LAGB represented 35.4 % of all bariatric procedures in the USA, in 2014, LAGB prevalence decreased to 9.5 % [5]. Although the exact reasons for the decline in this surgical procedure have not been studied directly, there are high rates of reoperation, weight regain and complications associated with LAGB [10]. Common complications that have been associated with LAGB are the following: port malfunctioning, band leakage, gastroesophageal reflux disease (GERD) and pouch dilation [3, 11, 12]. More severe complications include band erosion (BE), slippage and intra-gastric band migration [3, 11, 12].
Band erosion is a rare complication after LAGB that was first described in 1998 [13]. BE is associated with a broad spectrum of symptoms ranging from asymptomatic to non-specific abdominal pain, bloating, nausea, vomiting, infection of the port and weight regain [14]. The rate of BE has been reported to be 1.46 % [13], but it can range from 0.2 to 14 % across studies. [14, 15]. Even though LAGB procedures have decreased overall, given the number of patients who have had LAGB in the past, surgeons still need to develop expertise in treating these complications. This is particularly important for surgeons who have had less experience in dealing with band complications.
Controversies exist regarding the management, approach and timing for the removal of the lap band [3]. The key point in the treatment is the removal of the band. However, the approach to the removal of the band differs across various studies. The two most popular methods are laparoscopic and endoscopic removal of the band, but several case reports have described other hybrid procedures that combine endoscopy and laparoscopy with an intra-gastric port [16, 17]. Therefore, additional guidelines for how to address LAGB complications and BE in particular are needed. The aim of this current study is to describe the rate, clinical presentation and perioperative outcomes of BE at our institution and to provide overall recommendations regarding the diagnosis and management of BE based on our experience and that of the previous literature in this area.
Materials and methods
Study population
This study is a single-center, retrospective review of a prospectively maintained database. Data were collected from all consecutive patients who underwent a LAGB and band revisional surgeries at the University of Illinois Hospital and Health Sciences System from December 2008 to September 2015. The term “revisional surgery” includes all procedures performed in patients with a previous LAGB such as band repositioning/removal, port repositioning/removal and conversions to sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB).We also defined the term “gastric fistula” as the defect in the gastric wall produced by the erosion of the band into the stomach lumen.
We identified patients who underwent gastric band removal due to BE as a complication. The following variables were obtained from the electronic medical records at the University of Illinois Hospital and Health Sciences System: sex, age at primary surgery, BMI at primary surgery and at band removal, time between placement and removal of the band, preoperative symptoms, previous abdominal surgeries, diagnostic methods used, esophagogastroduodenoscopy (EGD) findings, intra-operative outcomes (approach, blood loss and operative time) and postoperative outcomes (length of hospitalization, complications, 30-day reoperations, readmissions and mortalities). Descriptive statistics were conducted using SPSS 22.0 (IBM, SPSS Statistics). This study was conducted with Institutional Review Board approval (2011-1104).
Results
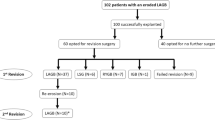
A total of 576 LAGBs were performed at our institution from December 2008 to September 2015. Of these, 420 (72.9 %) patients received LAGB placements alone with no additional procedures, 149 (25.9 %) patients had a LAGB with a hiatal hernia repair and 7 (1.2 %) patients underwent a LAGB over a previous RYGB. Overall, 194 LAGB revisional surgeries were performed from December 2008 to September 2015. Not all of the primary LAGB surgeries were performed at our institution. Data revealed that 80 (41.2 %) of the revisional surgeries were band removals due to slippage, pouch dilation and migration, 21 (10.8 %) were band repositions, 24 (12.4 %) were port complications that required either repositioning or removal due to infection, and 9 (4.6 %) were band removals due to BE. Finally, due to inadequate weight loss, 34 (17.5 %) patients underwent conversion to SG and 26 (13.4 %) patients underwent conversion to RYGB.
A total of nine patients underwent surgery for a BE at the University of Illinois Hospital and Health Sciences System. Out of these nine patients, eight had their primary LAGB placement at our institution and one patient received the primary LAGB at an outside institution. The rate of BE from patients who received their primary LAGB at our hospital was 1.39 % (n = 8).
Table 1 shows the demographics, weight characteristics and surgical timing of the nine patients who underwent surgery for a BE. The mean age at the time of the primary LAGB was 43.3 years old (SD = 11.8), the mean BMI at the time of the primary LAGB was 43.4 kg/m2 (SD = 5.6), and 100 % of the patients were female. The average time between the primary surgery and the removal of the band was 68.5 months (SD = 42.9). The mean BMI at the time of the removal of the band was 34.2 kg/m2 (SD = 5.9).
Table 2 summarizes the preoperative characteristics of the patients with a BE. As shown in this table, abdominal pain, nausea and/or vomiting were the most frequently mentioned symptoms. One patient was asymptomatic, and she was initially scheduled for a revisional surgery for conversion to a SG. During her revisional surgery, a BE was detected, the conversion to SG was not performed, and the band was instead removed. Out of the nine patients, six had at least one previous abdominal surgery in addition to the LAGB. With all patients, a preoperative or intra-operative EGD was conducted to assess the gastric fistula repair. Three patients also underwent an upper gastrointestinal (GI) fluoroscopy prior to the EGD which suggested a BE. The fluoroscopy revealed contrast flowing outside the band which is an unequivocal sign of BE (cascade sign). All BEs were partial.
Surgical technique
With all patients, a minimally invasive gastric band removal was performed either laparoscopically or robotically following the same surgical technique. Surgery commenced with a diagnostic laparoscopy. All patients showed significant subacute inflammation surrounding the band. In the first step, the band tubing was identified and followed until it reached the band. Dissection was guided following the tubing. Minimal dissection was conducted while exposing the band buckle when possible. Then the exposed band was transected and removed from around the stomach. The gastric fistula resulting from the BE was repaired in two layers with a 3.0 continued or interrupted absorbable suture. An intra-operative EGD was performed to rule out a leak or bleeding. In all patients, a Jackson-Pratt drain was placed in the abdomen next to the gastric repair. In four patients, an omentum patch was placed to reinforce the closure. As noted in Table 2, one patient presented with 95 % erosion of the band into the stomach. The band or buckle could not be identified from the abdominal cavity. In this case, an anterior gastrotomy was performed to remove the band from inside the stomach lumen. Then, the repair of the gastrotomy was performed with a double layer of an absorbable suture.
Table 3 shows the intra-operative and postoperative outcomes. As shown in this table, a laparoscopic band removal was used with six patients and in the remaining patients (n = 3), a robotic approach was used. All patients received clear liquids between the first and second postoperative day. None of the patients had a band replacement or a conversion to another bariatric procedure at the time of the removal of the eroded band. Overall, across the BEs, the mean operating time was a 119.6 min (SD = 47.7) and ranged from 70 to 236 min. The mean estimated blood loss was 13.3 cc (SD = 7.1) ranging from 5 to 20 cc, which was minimal. The mean length of hospitalization was 2.6 days (SD = 1.1) and ranged from 1 to 4 days. Hospitalization was prolonged in two patients: One patient had a port site abscess prior to the revisional surgery for the eroded band, and another patient, with a history of GERD, had pneumonia, resulting in a hospitalization of 3 and 4 days, respectively. Both of these patients also required antibiotic therapy. No other complications were registered. There were no 30-day reoperations, readmissions or mortalities.
Discussion
LAGB has been found to have good initial outcomes [14]. However, high rates of long-term complications are frequently observed in follow-up studies of patients with LAGB [6, 14]. For example, in one of these studies, 50 % of patients with a LAGB required a reoperation, 25 % experienced a major late complication, and 73 % of patients reported that they would not choose a LAGB again [6]. The number of LAGB procedures has also decreased drastically due to complications such as leak and slippage, as well as inadequate weight loss associated with LAGB [10]. Finally, prior studies have reported failure rates of LAGB between 2 and 70 %, with reported revision rates as high as 59 % [18].
Band erosion is a rare complication of LAGB. In this study, the rate of BE from patients who received their primary LAGB at our hospital was 1.39 % which is in the lower limit of the range previously described in literature [14, 15]. This incidence rate includes only patients who had their primary LAGB at our institution. We used the same method to calculate the incidence rate of BE that was used in a prior study [3]. It is also possible that some of the patients who had their primary LAGB at our institution went to an outside facility or surgeon when problems such as a BE arose from their LAGB. We acknowledge that this would mean an underestimation of the incidence rate of BE at our institution.
There are many theories about the causes of BE. These include: infection of the port, over-distension of the band and subsequent ischemia of the stomach, widespread dissection during the primary surgery with impaired blood supply and susceptibility to pressure ischemia, and serosal damage at the moment of the insertion of the band with posterior inflammatory reaction leading to erosion [19]. In our study, the mean time between surgery and BE was almost 5 years, which is considered a long time between the primary surgery and the BE complication. Due to the length of time that elapsed between the primary LAGB and the BE, it is likely that the erosion was related to a chronic inflammatory process associated with the introduction of a foreign body, rather than to any technical details of the surgical procedure itself, as BEs related to the surgical procedure would manifest sooner. However, there is a need to further investigate reasons for BE and the timing of this complication as it relates to the primary LAGB in future studies.
The experience of the surgeon in conducting primary LAGB is another important variable affecting the incidence rate of BE [3, 12]. Specifically, it has been suggested that technical details that occur during the surgery may improve the surgical outcomes, as there is a decrease in the rate of BE as more LAGBs are performed [3, 12]. This idea was discussed in a study by Cherian et al. [3]. In that study, the authors reported an overall incidence of BE of 1.96 % (17 out of 875 cases). However, in the first 300 cases, there was a BE incidence rate of 4.7 % (n = 14) and a 0.53 % (n = 3) BE incidence rate in the last 565 patients. The fact that 83 % of the BEs in that study occurred in the first 300 patients appeared to support the notion that BEs decreased as more surgeries were performed [3].
Recommendations regarding the diagnosis and management of band erosion
Band erosion is one of the most severe complications of LAGB, and some patients present with peritonitis and sepsis [19]. Figure 1 illustrates a flowchart of our recommendations regarding the diagnosis and management of BE, based on our experience and previous research in this area. As shown in Fig. 1, clinically, patients present with abdominal pain, nausea, vomiting and port infection, and the diagnosis must be confirmed with an EGD. Information provided by an EGD is a very important part of determining the treatment approach. In the current study, all patients received a diagnostic EGD and it was also a useful intra-operative tool to assess the gastric repair. The treatment for BE must always involve the removal of the band. However, controversy exists in the literature and in clinical practice regarding the timing and approach to manage this complication [16]. Many factors influence this process, including the clinical presentation, the extent of the BE, the equipment available and the surgeon’s experience. Usually, it is recommended to wait at least 3 months after the removal of the band to replace it [13]. We do not recommend one stage removal and replacement of the band because RYGB and SG have been shown to have better outcomes than LAGB and the recurrence rate of BE is also high [15, 20].
There are several key issues regarding the management of BE that relate to the timing of the removal of the LAGB and to the retrieval method of the LAGB. With respect to the timing of the removal of the eroded band, there are two approaches that have been discussed in the literature. In the first approach, the band is removed as soon as the diagnosis made. The rationale is that patients can benefit from immediate treatment to improve their symptoms and lower the risk of further complications (peritonitis, hemorrhage and band migration) [3, 11]. This approach also allows patients to restore their daily activities, reduce days absent from work and reduce additional medical consultations. In the second approach, the band is removed endoscopically. In this case, treatment is sometimes delayed when the diagnosis is premature and the band is minimally eroded into the lumen. A delay in treatment leads to further erosion of the band allowing endoscopical retrieval.
As shown in Fig. 1, the second issue related to the management of BE concerns the retrieval method of the band, which is done through a minimally invasive method (laparoscopic or robotic), endoscopically, or through the use of hybrid techniques. In our opinion, for partial BEs, a minimally invasive approach appears to be safe and enables an immediate resolution of the complication. In this series of patients, the gastric fistula was always closed and in four cases, an omentum patch was used to reinforce the suture. An intra-operative EGD was used to assess the gastric repair. The intra-operative EGD is a very useful tool for the surgical management of BE. Few case reports describe fistula repair using only the omentum patch [3], but there is no evidence to support this type of closure. Usually, a hermetic gastric defect closure is recommended. Overall, we believe that with experienced surgeons, laparoscopic removal is feasible and can effectively solve the problem of BE, with low postoperative complication rates, short operative times and length of hospitalization.
As also shown in Fig. 1, an endoscopic retrieval of the band is an alternative approach that can be used in select cases. Previous studies have noted that at least 50 % of the circumference of the band must be eroded into the gastric lumen for the endoscopic removal to be possible [9, 17]. Other authors report that almost a complete erosion of the band is needed before it can be endoscopically removed [11]. In many cases, treatment is delayed or even a larger gastrotomy at the site of the erosion is necessary to remove the band endoscopically, with more than one attempt necessary for a successful retrieval [21]. These procedures are all done with general anesthesia [17]. Furthermore, the buckle portion of the band must be in the lumen for endoscopic removal, which may also decrease the chance of successful removal of the band. The most common causes of failure to retrieve the band are complications with the cutting device and firm fixation of the band outside the gastric wall by adhesions [14]. In addition, the device called Gastric Band Cutter® (Agency for Medical Innovation GmbH, Gotzis Austria) [14] used to cut the band is not approved by the Food and Drug Administration in the USA, so it is not available in most bariatric centers in the USA [16]. Furthermore, as shown in Fig. 1, endoscopic removal of bands requires an experienced endoscopic team with advanced skills and an open surgical procedure to remove the port. Some studies reported a high success rate with band removals using an endoscopic approach, but most of these studies also include bands that migrated into the stomach lumen, which is the most advanced stage of BE [12]. Dogan et al. [12] reviewed two studies [19, 22] and reported that across these studies, the success rates of endoscopic band removal ranged from 77 to 92 % and the complication rates ranged from 0 to 10 % [12, 19, 22]. Moreover, with an endoscopic approach, the most frequently reported complication was symptomatic pneumoperitoneum [12, 19, 22]. There are also descriptions of endoscopic removals that require an initial extension of the gastric fistula with a posterior procedure a week later to cut and remove the band [21]. In our series, the endoscopic approach was not used for band removals because there were no complete erosions and the Gastric Band Cutter® is not available at our institution.
Finally, there are also case reports of hybrid techniques to retrieve the LAGB. These procedures involve the use of an endoscopy and an intra-gastric laparoscopic port to cut and remove the band, and then place a gastrostomy tube through the opening in the stomach which remains there for 2 weeks [16]. Other researchers describe the use of both endoscopy and conventional laparoscopy to facilitate the endoscopic retrieval of a gastric band [17]. This approach enables the surgeon to close the fistula and cut the connecting tube [17]. However, in our opinion, the above-cited procedures may be cumbersome and are difficult to replicate.
In summary, as shown in Fig. 1, BEs usually present with abdominal pain, nausea, vomiting or port infection. The diagnosis must always be confirmed with an EGD. For partial BEs, we recommend minimally invasive (laparoscopic or robotic) removal of the band. When the buckle or band is visible in the abdominal cavity, the band can be cut and removed with posterior repair of the gastric fistula. If it is not possible to identify the buckle or band, the latter can be removed through an anterior gastrotomy with posterior closure of the gastrotomy. In the literature, endoscopic removal of partial BEs is recommended only when the endoscopic team is experienced and has advanced skills. As also shown in Fig. 1, when the BE is complete, the band can be removed endoscopically. If endoscopy fails, the removal must be performed with a minimally invasive approach.
Conclusions
Band erosion is one of the most severe complications of LAGB. Based on our experience and that of the previous literature, either endoscopic or surgical intervention is needed to remove the band to resolve this complication. In this retrospective review of patients who had a BE, even those with multiple previous abdominal surgeries and those with various stages of band erosion appeared to benefit from minimally invasive (laparoscopic or robotic) removal of the band. The minimally invasive approach also provided us with the opportunity to repair the fistula, and it was associated with a prompt recovery with very little morbidity. In general, it is recommended that the band be removed at the time of the BE diagnosis in order to avoid further complications and progression of the erosion. It is also recommended that endoscopic band removal be utilized with patients who have a complete BE and migration into the gastric lumen or partial BEs managed by an experienced endoscopic team with advanced skills. Additional research into the reasons for BE and its management is needed in order to further prevent and treat this complication among patients who have undergone a LAGB.
References
Echaverry-Navarrete DJ, Maldonado-Vazquez A, Cortes-Romano P, Cabrera-Jardines R, Mondragon-Pinzon EE, Castillo-Gonzalez FA (2015) Gastric band erosion: alternative management. Cir Cir 83:418–423
Sturm R, Hattori A (2013) Morbid obesity rates continue to rise rapidly in the United States. Int J Obes 37:889–891
Cherian PT, Goussous G, Ashori F, Sigurdsson A (2010) Band erosion after laparoscopic gastric banding: a retrospective analysis of 865 patients over 5 years. Surg Endosc 24:2031–2038
Solomon H, Liu GY, Alami R, Morton J, Curet MJ (2009) Benefits to patients choosing preoperative weight loss in gastric bypass surgery: new results of a randomized trial. J Am Coll Surg 208:241–245
Ponce J, Nguyen NT, Hutter M, Sudan R, Morton JM (2015) American Society for Metabolic and Bariatric Surgery estimation of bariatric surgery procedures in the United States, 2011–2014. Surg Obes Relat Dis 11:1199–1200
Kodner C, Hartman DR (2014) Complications of adjustable gastric banding surgery for obesity. Am Fam Physician 89:813–818
Pentin PL, Nashelsky J (2005) What are the indications for bariatric surgery? J Fam Pract 54:633–634
O’Brien PE (2010) Bariatric surgery: mechanisms, indications and outcomes. J Gastroenterol Hepatol 25:1358–1365
Kohn GP, Hansen CA, Gilhome RW, McHenry RC, Spilias DC, Hensman C (2012) Laparoscopic management of gastric band erosions: a 10-year series of 49 cases. Surg Endosc 26:541–545
Trujillo MR, Muller D, Widmer JD, Warschkow R, Muller MK (2015) Long-term follow-up of gastric banding 10 years and beyond. Obes Surg 26:581–587
Aarts EO, van Wageningen B, Berends F, Janssen I, Wahab P, Groenen M (2015) Intragastric band erosion: experiences with gastrointestinal endoscopic removal. World J Gastroenterol 21:1567–1572
Dogan UB, Akin MS, Yalaki S, Akova A, Yilmaz C (2014) Endoscopic management of gastric band erosions: a 7-year series of 14 patients. Can J Surg 57:106–111
Egberts K, Brown WA, O’Brien PE (2011) Systematic review of erosion after laparoscopic adjustable gastric banding. Obes Surg 21:1272–1279
Mozzi E, Lattuada E, Zappa MA, Granelli P, De Ruberto F, Armocida A, Roviaro G (2011) Treatment of band erosion: feasibility and safety of endoscopic band removal. Surg Endosc 25:3918–3922
Brown WA, Egberts KJ, Franke-Richard D, Thodiyil P, Anderson ML, O’Brien PE (2013) Erosions after laparoscopic adjustable gastric banding: diagnosis and management. Ann Surg 257:1047–1052
El-Hayek K, Timratana P, Brethauer SA, Chand B (2013) Complete endoscopic/transgastric retrieval of eroded gastric band: description of a novel technique and review of the literature. Surg Endosc 27:2974–2979
Rodarte-Shade M, Barrera GT, Arredondo JF, Diaz RR (2013) Hybrid technique for removal of eroded adjustable gastric band. JSLS 17:338–341
Carandina S, Tabbara M, Bossi M, Helmy N, Polliand C, Barrat C (2014) Two stages conversion of failed laparoscopic adjustable gastric banding to laparoscopic roux-en-y gastric bypass. A study of one hundred patients. J Gastrointest Surg 18:1730–1736
Chisholm J, Kitan N, Toouli J, Kow L (2011) Gastric band erosion in 63 cases: endoscopic removal and rebanding evaluated. Obes Surg 21:1676–1681
Hutter MM, Schirmer BD, Jones DB, Ko CY, Cohen ME, Merkow RP, Nguyen NT (2011) First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg 254:410–420
Campos JM, Evangelista LF, Galvao Neto MP, Ramos AC, Martins JP, dos Santos MA, Ferraz AA Jr (2010) Small erosion of adjustable gastric band: endoscopic removal through incision in gastric wall. Surg Laparosc Endosc Percutan Tech 20:e215–e217
Neto MP, Ramos AC, Campos JM, Murakami AH, Falcao M, Moura EH, Evangelista LF, Escalona A, Zundel N (2010) Endoscopic removal of eroded adjustable gastric band: lessons learned after 5 years and 78 cases. Surg Obes Relat Dis 6:423–427
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Pablo Quadri, Raquel Gonzalez-Heredia, Mario Masrur, Lisa Sanchez-Johnsen and Enrique Elli have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Quadri, P., Gonzalez-Heredia, R., Masrur, M. et al. Management of laparoscopic adjustable gastric band erosion. Surg Endosc 31, 1505–1512 (2017). https://doi.org/10.1007/s00464-016-5183-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-5183-4