Abstract
Background
Sponsored by the European Commission, the FP7 STIFF-FLOP project aimed at developing a STIFFness controllable Flexible and Learn-able manipulator for surgical operations, in order to overcome the current limitations of rigid-link robotic technology. Herein, we describe the first cadaveric series of total mesorectal excision (TME) using a soft and flexible robotic arm for optic vision in a cadaver model.
Methods
TME assisted by the STIFF-FLOP robotic optics was successfully performed in two embalmed male human cadavers. The soft and flexible optic prototype consisted of two modules, each measuring 60 mm in length and 14.3 mm in maximum outer diameter. The robot was attached to a rigid shaft connected to an anthropomorphic manipulator robot arm with six degrees of freedom. The controller device was equipped with two joysticks. The cadavers (BMI 25 and 28 kg/m2) were prepared according to the Thiel embalming method. The procedure was performed using three standard laparoscopic instruments for traction and dissection, with the aid of a 30° rigid optics in the rear for documentation.
Results
Following mobilization of the left colonic flexure and division of the inferior mesenteric vessels, TME was completed down to the pelvic floor. The STIFF-FLOP robotic optic arm seemed to acquire superior angles of vision of the surgical field in the pelvis, resulting in an intact mesorectum in both cases. Completion times of the procedures were 165 and 145 min, respectively. No intraoperative complications occurred. No technical failures were registered.
Conclusions
The STIFF-FLOP soft and flexible robotic optic arm proved effective in assisting a laparoscopic TME in human cadavers, with a superior field of vision compared to the standard laparoscopic vision, especially low in the pelvis. The introduction of soft and flexible robotic devices may aid in overcoming the technical challenges of difficult laparoscopic procedures based on standard rigid instruments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Although laparoscopic resection of colon cancer is recently gaining acceptance [1–4], the role of laparoscopy in the treatment of rectal cancer is still controversial. Excellence of surgical technique is of particular relevance in the treatment of rectal cancer. Routine excision of the intact mesorectum during resection of cancers of the middle and lower rectum has resulted in a consistent reduction in local recurrences [5] and in an increase in long-term survival rates [6]. At present, however, open surgery is still considered the treatment of choice for elective rectal resection for malignant diseases.
The COLOR II trial, a non-inferiority phase 3 multicentric trial, showed how laparoscopic TME resulted in similar safety, resection margins, and completeness of resection to that of open surgery [7]. Furthermore, laparoscopic surgery in patients with rectal cancer was associated with rates of locoregional recurrence- and disease-free and overall survival at 3 years similar to those for open surgery [8]. On the other hand, the ACOSOG [9] and the ALaCaRT [10], two randomized non-inferiority phase 3 trials, both failed to meet the criterion for non-inferiority for pathologic outcomes in stage II and III rectal cancer. The primary end point was a composite of oncological factors indicating an adequate surgical resection, defined as meeting all the following criteria: (1) complete total mesorectal excision, (2) a clear circumferential margin (1 mm), and (3) a clear distal resection margin (1 mm).
We recently conducted a systematic review and meta-analysis [11] of available data, which proves that on the basis of evidence of both randomized and prospective matched series, laparoscopic rectal resection appears to have clinically measurable short-term advantages in selected patients with primary resectable rectal cancer. The main finding of the cited meta-analysis was a significant reduction in mortality in the laparoscopic group as compared to the open-surgery group. Furthermore, the overall incidence of postoperative complications was also significantly lower in the laparoscopic group, with a relative risk (RR) of 0.81. The analysis of all included studies showed a clear advantage for laparoscopy in the specific analysis of both surgical and medical complications. Therefore, it can be concluded that, although technically demanding, laparoscopic rectal resection is safe and results in faster recovery. This conclusion remains accurate when the analysis is restricted to only extra-peritoneal lesions or mid–low rectal cancers [12], which entails TME with or without colo-anal anastomosis or abdomino-perineal resection with anal amputation, both approaches being even more technically challenging.
Also, laparoscopy does not impair the oncologic results of surgery as we demonstrated in a further systematic review and meta-analysis [13]. Based on the evidence from the randomized controlled trials (RCTs) and non-RCTs examined in this systematic review, the short-term benefit and oncological adequacy of laparoscopic rectal resection appears to be equal to open surgery, with some evidence potentially pointing to comparable long-term outcomes and oncological adequacy in selected patients with primary resectable rectal cancer.
Regardless of these studies, minimally invasive surgery for rectal cancer fails to affirm its superiority in routine use due to the technical challenges that force long training, stressful procedures, and careful patient selection. It has been advocated that robotic technology might be of some help in reducing challenges and learning curves.
Standard robotic platforms, such as da Vinci Surgical System (Intuitive Surgical, CA, USA,—www.intuitivesurgical.com), are quite expensive and their true potential, in the management of rectal tumours, still needs to be verified, despite the possible advantages of providing greater dexterity and better ergonomics to the surgeon [14]. Short-term outcomes of the RCT comparing RObotic and LAparoscopic Resection for Rectal Cancer (ROLARR) demonstrated no significant differences between the laparoscopic and robotic group in the conversion rates to open surgery, in intraoperative and postoperative complications, and in the rate of positive circumferential margins [15]. The results of this large RCT suggest that the use of the current robotic technology does not help to improve the outcomes observed after conventional laparoscopic surgery.
Robotic instruments have the potential to allow an improved manoeuvrability in confined spaces, considerably simplifying the procedure. For this reason, flexible devices for minimally invasive surgery purpose have been developed in research. Nevertheless, no specific devices have been developed to accomplish surgical tasks in the rectum. Deflectable tip laparoscopes, for example, may provide a better field of vision in the narrow pelvis during rectal resections compared to standard rigid laparoscopes. Recent research on soft and stiffness-controllable robotics has clearly shown the potential of such robotic systems for surgical applications [16–18]. Research has recently focused on creating flexible robotic platforms equipped with highly dexterous arms capable of operating in different body districts, such as heart or throat [19–21], brain [22, 23] or on abdominal organs, through a single port access [24, 25]. In addition, also flexible endoscopes with enhanced functionalities have been sometimes employed as flexible guides for instruments targeting abdominal organs through a natural orifice access [26, 27]. Both highly articulated surgical instruments and flexible endoscopes make use of their body structures to guide the tip of the instrument in order to reach the surgical site. Therefore, the interactions with tissue are avoided or limited when the instrument is actively guided (i.e. in a highly articulated system), while they are not monitored for the case of endoscopes [28]. In order to manage the interaction between organs (and generally tissue) and the body of the instrument (not only the end-effector tool), a compliant instrument structure is desirable. This compliancy, however, should coexist with a capability to increase the structure’s stiffness when considerable forces have to be exerted by the tools integrated with the instrument tips (i.e. to retract tissue or organs or to accomplish surgical tasks with the end effector) [29, 30].
In 2011, based on these assumptions and taking inspiration from nature, we conceived the notion to considerably change the design of a robotic surgical instrument, taking into consideration the importance of generating a soft device, which when circumstances dictate, can be appropriately stiffened. Our inspiration came from the tentacles of an octopus. The project was funded by the European Commission within the Seventh Framework Programme and started on 1 January 2012.
As this project has evolved over the last four years, we developed a robotic arm containing an optical camera, with a reasonable dimension, able to be inserted through a standard commercially available 15-mm trocar to facilitate testing on a human model. To date, abdominal surgery with the aid of soft robotic devices has not been described in the literature. The aim of this study is to demonstrate the feasibility of laparoscopic TME with the aid of soft and flexible robotic optics in a cadaveric model.
Methods
Developing the prototype included manufacturing the flexible modules and incorporation of sensing capabilities, force feedback, navigation control, as well as a user interface and advanced algorithms to enable integration of all of the above. At first, large-scale prototypes were developed in order to test the concept on bench-top models. A set of STIFF-FLOP arm prototypes with a diameter of 24 mm were manufactured, consisting of multiple soft, pneumatically actuated three-chamber segments [31]. Additional chambers are integrated within the segments to allow their stiffening, employing an approach based on the concept of granular jamming (Fig. 1). Prototypes with two and three segments, respectively, were created and several large-scale human abdominal cavity models were manufactured for testing the system [32] (Figs. 2, 3). The STIFF-FLOP segments are actuated using pressure regulators, which are controlled by RoNeX boards. (RoNeX boards are produced by London-based company Shadow Robotics and were developed as part of the STIFF-FLOP project to facilitate hardware integration.) In a similar fashion, the stiffening chambers are interfaced via valves also controlled by RoNeX boards, applying a vacuum to the granules in the chambers which in turn generate a stiffening behaviour for the STIFF-FLOP arm.
Computer model design of one STIFF-FLOP arm segment. From the left Section view of the segment showing the arrangement of the chambers (pneumatic and stiffening); segment in the rest position (no pressure is supplied to the chambers); bending of the segment due to the pressurization of one pneumatic chamber (in dark blue); elongation of the segment due to the simultaneous pressurization of all the chambers. The stiffening mechanism can be activated by controlling the level of vacuum in all the stiffening chambers (in red), once the desired position of the segment is reached (Color figure online)
Sensors are embedded in the STIFF-FLOP modules to measure interaction forces (between the robot and its environment) and the robot’s configuration. In this context, each segment is equipped with a three-axis force/torque (F/T) sensor and a three-DoF bending sensor. To augment the configuration (pose) tracking, two external sensors, a laparoscopic camera and an NDI Aurora magnetic tracker (NDI International Headquarters, Waterloo, Ontario, Canada), are employed. To this end, sets of markers are attached at various locations along the STIFF-FLOP arm. A commercial robotic arm (Schunk GmbH & Co. KG, Hamburg, Germany) is attached to the base of the STIFF-FLOP arm, outside of the abdomen, to move the STIFF-FLOP arm in and out through the trocar and to position and orientate the base of the STIFF-FLOP arm as required. Input from the magnetic trackers ensures that the pivot point of the trocar and the robotic arm are identical. This approach assures that the STIFF-FLOP arm is always inserted along the central axis of the trocar port, pitching and yawing about the trocar insertion point. Advanced control and navigation techniques have been developed and are integrated computing the inverse kinematics for the extended kinematic chain of the Schunk arm and the STIFF-FLOP arm in real time, based on the inputs from the various sensors. This allows the surgeon to control the tip of the extended robot arm in tool space without the need to control the proximal part of the arm.
A newly developed user interface, based on a Delta robot design [33], is used to move and position the tip of the STIFF-FLOP arm inside the abdomen (Fig. 4). In addition to the standard visual feedback from a laparoscopic camera, a real-time 3D visualizer showing 3D views of the STIFF-FLOP modules is also available. Signals obtained from the F/T sensors are fed back to the user interface console providing the operator with force feedback, effectively resisting the operator’s motion when the robot is in physical contact with the environment. The end effector of these prototypes was equipped with different tools, including a monopolar hook and a gripper. Successful usage of these tools in realistic environments was demonstrated.
Once functionality was proven in the ex vivo setting, a thinner prototype capable of passing through a standard 15-mm trocar cannula was developed for experiments in human cadavers. This fully integrated prototype consists of miniature pneumatically actuated segments, a positioning device, and a camera at the tip. The consortium successfully managed to scale down the overall system dimensions to a 14.3-mm-diameter soft robot, capable of being inserted into the human body via a commercially available trocar port. Scaling down the dimensions of the prototype compelled us to forgo all of the sensing capabilities except the camera, so that controlling the tip of the soft robot was possible by separately controlling the two robots’ modules with the two joystick input device. This control allows each robot module to bend in all the directions and also elongate longitudinally along the central axis. The entire soft robot is equipped with a 4 mm in diameter centre-free lumen, which allows the passage of the electrical wires needed for the laparoscopic miniaturized optic system positioned at the tip of the robot. The employed MD-T1003L-65 optics [Misumi New Taipei City, Taiwan (R.O.C.)] measure 3.8 mm in diameter and 12 mm in length; the optics are integrated with an illumination system (four LEDs) and are connected via USB to a computer system. The STIFF-FLOP camera robot was attached to a rigid hollow shaft (10 mm of diameter), which was connected to the operative table by means of an anthropomorphic arm with three ball-shaped joints (KLS Martin GmbH + Co. KG, Freiburg, Germany). This arm could be manually adjusted during the operation for a proper positioning of the base of the STIFF-FLOP robot. The main objective of the test was to validate that the architecture of the system was compatible with human anatomy for laparoscopic TME and to determine whether the softness, flexibility, and dexterity of the soft-robot-based optics could represent a potential improvement compared to standard rigid laparoscopic instrumentation.
Operative technique
A 1-day session on human cadavers took place at the Institute for Medical Science and Technology (IMSaT), Dundee, Scotland. The aim of this session was to prove the feasibility of the use of the 14-mm STIFF-FLOP camera robot whilst performing a minimally invasive laparoscopic TME. The team of engineers installed the entire system, including the software and the STIFF-FLOP camera robot that was secured to the operative table via a Martin arm. The surgical team (AA, YM, MEA\) used two human cadavers previously selected.
The study was performed on two cadavers made available at the Centre for Anatomy and Human Identification, University of Dundee, and prepared according to the method described by Thiel. The Thiel embalming method for cadaver preservation is a technique, which relies on a mixture of salt compounds and very low amounts of volatile formaldehyde and formalin which effect fixation of tissue with a number of unique properties. Cadavers preserved with this method have no detectable odour and demonstrate a lifelike flexibility of body parts, excellent colour preservation of muscle, viscera, and vasculature, and superior antimicrobial preservation properties. Due to this preservation of lifelike qualities, soft-embalmed cadavers are excellent models for training in surgical, diagnostic, and interventional procedures as well as a model for research and development of new surgical devices. In our experiment, the BMI for the cadavers were 25 and 28 kg/m2, respectively.
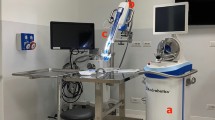
Prior to starting the session, each cadaver was positioned and safely secured to a mobile operating table, and all instrumentation was thoroughly checked. For the duration of surgery, the cadavers were strapped to a Maquet surgical table (Maquet Holding B.V. & Co. KG, Rastatt, Germany) and draped in standard surgery gowns in preparation for the surgical intervention. At the beginning of each test, four trocars were inserted: one 15-mm trocar on the median line about 2 cm above the umbilicus, through which the flexible STIFF-FLOP camera was inserted, and the other three 5/12-mm trocars in the left flank, right flank, and right iliac regions, respectively. At that point, the consistence of both bowel and mesocolic fatty tissue was carefully checked. An additional 10-mm trocar was placed in the left upper quadrant, posterior to the STIFF-FLOP camera, to obtain an overview vision by means of a standard rigid 30° laparoscopic 10-mm camera (Fig. 5). Two monitors were used to follow the procedure: one was connected to the rigid standard laparoscopic camera, while the other one was connected to the flexible STIFF-FLOP camera. The gross anatomy of the abdominal cavity and the compliance of the abdominal wall to the CO2 insufflation were evaluated. Camera images were recorded at all times at a standard rate of 24 frames per second for subsequent analysis.
The dissection was then initiated, using sharp scissor dissection and standard laparoscopic instruments. The dissection was carried out proximally in an infra-mesocolic dissection plane identifying the avascular plane caudal and cranial to the inferior mesenteric artery. The inferior mesenteric artery (IMA) was then divided using standard titanium clips and scissors. Then the posterior mesorectum was identified and dissection was continued in the pre-sacral avascular space to the level of the pelvic floor. The left and right iliac vessels and ureters were identified at this point. The lateral dissection plane was then thinned out with anterior blunt traction and dissection. The anterior dissection was then performed with sharp dissection posterior to Denonvilliers’ fascia. The seminal vesicles and the right and left ureters were identified and spared from injury. The anterior dissection plane was continued laterally, further thinning out the remaining lateral stalks, taking care to preserve the lateral pelvic nerve bundles. The lateral stalks were divided, moving the optics from one side to the other, over the rectum keeping the surgical field in the optimal line of vision. The circumferential mobilization of the rectum was then completed. The integrity of the specimen, with particular attention to the mesorectal fascia, was evaluated laparoscopic ally.
Results
Both cadavers were operated on the same day by a team of three surgeons. While one surgeon manipulated the STIFF-FLOP camera through a controller independently moving each of the two modules by means of a dedicated joystick-based input device, the second surgeon performed the TME and the third surgeon was in charge of documentation using the standard laparoscope. The control of the STIFF-FLOP camera was conducted by using only the visual feedback provided by the embedded optics, as no other sensors were integrated in the prototype used for the presented cadaver tests (video 1).
The first step of the procedure included the medial dissection of the mesocolon of the sigmoid and descending colon, and the identification and division of the vessels (Fig. 6). The use of the STIFF-FLOP camera allowed the surgeon to clearly visualize the inferior mesenteric vessels and the autonomic nerves that were subsequently spared from injury. After the completion of the vessel division, the sigmoid mesocolon was completely dissected. Then, the instruments were moved down to the pelvis to start the TME under direct visualization of the STIFF-FLOP camera. The surgeons performed first the posterior dissection of the mesorectum down to the pelvic floor. The ability to smoothly follow the sacral curve due to the flexibility of the manipulator and the magnified vision provided by the STIFF-FLOP camera, allowed the surgeons to perform a very precise dissection of the posterior part of the mesorectum (Figs. 7, 8). The mesorectal excision was then completed laterally on both right and left sides of the rectum as well as anteriorly (Fig. 9). This step of the procedure was performed quite easily due to the flexibility of the modules that allowed the surgeons to achieve a magnified vision of the mesorectum and adjacent structures. The same procedure was performed on both human cadavers, demonstrating the ease of use of the system as well as its robustness of operation over many hours.
A complete TME dissection was completed in both cadavers resecting the mesorectal fascia down to the pelvic floor. The STIFF-FLOP robotic optics assured a sufficient visualization of the surgical field in both cases, so that an intact mesorectum was obtained at completion of both cases. The STIFF-FLOP robotic arm was inserted with no perceivable difficulty, through a standard 15-mm trocar and without limitation of movement in and out. The camera was cleaned approximately twice for each procedure in the standard approach, i.e. the arm was taken out for cleaning. Manipulation of the dual-joystick input device was achieved easily following a few minutes of practice and understanding of the movements, using the images from the laparoscopic camera as a navigational aid. Following this minimal training, control of the STIFF-FLOP camera was achieved without difficulty. Completion times of the procedure were 165 and 145 min, respectively. No intraoperative complications were recorded. No technical failures were registered.
Discussion
Laparoscopic low anterior resection with TME is a feasible approach for the surgical management of rectal cancer; however, it is technically challenging. Despite the evident benefits for the patient’s outcome [10, 11], this technique has not been generally adopted and has a limited routine application. Robotic technology, thus far has failed to deliver significant benefits in gaining better outcomes as compared to standard laparoscopy, both in terms of technical challenges for the operator and of clinical benefits for the patient [13]. Current attention is on improving training, both for standard laparoscopy and for robotics. We believe that adding the new concept of flexible robotic arms on top of the existing robotic technology may be the impetus needed to reduce the “human” factor which influences the end results using current “rigid” technology.
For this reason, in 2011, we envisioned a soft and flexible robot whose characteristics should theoretically allow better flexibility in narrow spaces, such as the pelvis. The project had a lifetime of four years, and allowed us to develop a modular technology, made of soft and flexible segments, characterized by the capability to bend under pneumatic inflation of dedicated chambers, as well as to become more stiff when required employing the concept of granular jamming of dedicated chambers integrated with the large-scale STIFF-FLOP arm prototypes. All movements are piloted by means of input devices based on a modified Delta robot design or dual joysticks. The STIFF-FLOP arm is designed to allow unconstrained and independent movement of each module, bending in all directions away from the longitudinal axis of each module, achieving a wide range of motions and a large workspace. Aside from module deflection, module elongation can also be achieved. The Delta robot input device allows for determination of the exact spatial coordinates the operator aims for the instrument’s tip position and orientation, while, similarly to the octopus tentacle, the required shape of the arm is achieved automatically via software. This adjustment is possible due to the presence of several sensors placed along the arm, which allow a fine control of the position of each module, as well as other force sensors that when used in conjunction provide a visual representation of the robot arm and haptic feedback to the operator through the Delta robot input device. These unique features are extremely innovative compared to flexible devices now available for surgery. The flexibility of currently used devices, both robotic and laparoscopic, is limited to the tip of the instrument, which maintains a rigid haulm unable to follow the curved lines of the human body. The possibility to have a modular stiff, but at the same time soft and flexible arm when necessary, could potentially allow to easily reach any position and direction in the abdomen or the chest.
In principle, these characteristics should overcome most of the current limitations of the available surgical robots, which are constrained by a rigid architecture. In order to downsize the robotic arm to a standard trocar size for the cadaver study, we decided to forgo the sensing capabilities for the time being and test the concept of the soft and flexible arm in a sensor-less fashion. For testing, the miniaturized STIFF-FLOP robot was attached to a rigid shaft connected to the operative table by means of an anthropomorphic arm and was controlled by means of a device equipped with a double joystick. The system was tested on human cadavers to evaluate and show feasibility and appropriateness of its geometry and function.
With the STIFF-FLOP arm equipped with frontal optics, a TME was completed in two consecutive cases by means of standard laparoscopic instruments. In both cases the procedure lasted less than 3 h, and neither intraoperative complications nor technical failures were registered. In particular, the capability of the STIFF-FLOP arm to enter the pelvis sliding either along the sacral bone, or getting across the rectum was appreciated, both of which are, in general, critical steps during a TME procedure. This unique ability allowed for close-up visualization of the surgical field by the surgeon when operating, which was more than sufficient to prove the concept and the correct geometry of the device.
The feasibility and safety of TME under soft and flexible robotic optics control, has now been demonstrated in human cadavers. Compared to current minimally invasive abdominal approaches (including robotic surgery), the authors have observed improved low-pelvic visualization, particularly during the posterior and left lateral dissection for TME. However, definitive conclusions for or against improved visualization are limited to this cadaveric series and will need further evaluation in a clinical setting. In particular, the system should be compared with the latest version of 3D high-definition laparoscope with a deflectable tip, which may provide an even better field of vision in the narrow pelvis during TME.
The strengths and the weaknesses of the optics were discussed between the two tests and at the end of the whole session. Pros of the robotic system included good image quality and stable control of the movements of the optics using the dual-joystick input device. Technical aspects requiring improvement are strictly related to device control, which needs to be more intuitive rather than separately controlling the modules by the joystick; thus, the approach used for the large prototype of the STIFF-FLOP arm (employing an inverse kinematics based approach) should be adopted. Embedding sensors in the prototype used for the cadavers and computing inverse kinematics in real time will allow the use of the modified Delta robot to control the pose (position as well as orientation) of the system, which in turn should improve on this issue of user-friendliness for robot navigation and tip positioning. At the same time, a prototype equipped with a gripper at the tip as well as a prototype equipped with a monopolar coagulator has been developed. This would allow testing the possibility of a complete soft and flexible robotic procedure, which will be attempted shortly.
Conclusions
This is the first report of a laparoscopic abdominal procedure performed with the aid of soft and flexible robotic technology. The results of this session suggest that the capability of the STIFF-FLOP manipulator allows performing a technically challenging procedure like a laparoscopic TME with a high level of precision and accuracy due to the optimization of the view of the surgical field. The safety and oncologic outcomes of TME using the current platform need to be further investigated in the setting of carefully conducted human cadaver tests first, followed by clinical trials.
References
Lacy AM, García-Valdecasas JC, Delgado S, Castells A, Taurá P, Piqué JM, Visa J (2002) Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet 359:2224–2229
Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM, MRC CLASICC trial group (2005) Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 365:1718–1726
Clinical Outcomes of Surgical Therapy Study Group (2004) A comparison of laparoscopic ally assisted and open colectomy for colon cancer. N Engl J Med 350:2050–2059
Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, Lacy AM, Colon Cancer Laparoscopic or Open Resection Study Group (COLOR) (2005) Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol 6:477–484
Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK (1998) Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978–1997. Arch Surg 133:894–899
Ries LA, Wingo PA, Miller DS, Howe HL, Weir HK, Rosenberg HM, Vernon SW, Cronin K, Edwards BK (2000) The annual report to the nation on the status of cancer, 1973–1997, with a special section on colorectal cancer. Cancer 88:2398–2424
van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, Bonjer HJ (2013) Colorectal cancer laparoscopic or open resection II (COLOR II) study group. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 14(3):210–218
Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES, Lacy AM, Bemelman WA, Andersson J, Angenete E, Rosenberg J, Fuerst A, Haglind E, COLOR II Study Group (2015) A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 372(14):1324–1332
Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas M, Peters WR Jr, Maun D, Chang G, Herline A, Fichera A, Mutch M, Wexner S, Whiteford M, Marks J, Birnbaum E, Margolin D, Larson D, Marcello P, Posner M, Read T, Monson J, Wren SM, Pisters PW, Nelson H (2015) Effect of laparoscopic-assisted resection versus open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA 314(13):1346–1355
Stevenson AR, Solomon MJ, Lumley JW, Hewett P, Clouston AD, Gebski VJ, Davies L, Wilson K, Hague W, Simes J, ALaCaRT Investigators (2015) Effect of laparoscopic-assisted resection versus open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA 314(13):1356–1363
Arezzo A, Passera R, Scozzari G, Verra M, Morino M (2013) Laparoscopy for rectal cancer reduces short-term mortality and morbidity: results of a systematic review and meta-analysis. Surg Endosc 27:1485–1502
Arezzo A, Passera R, Scozzari G, Verra M, Morino M (2013) Laparoscopy for extraperitoneal rectal cancer reduces short-term morbidity: results of a systematic review and meta-analysis. United Eur Gastroenterol J 1:32–47
Arezzo A, Passera R, Salvai A, Arolfo S, Allaix ME, Schwarzer G, Morino M (2015) Laparoscopy for rectal cancer is oncologically adequate: a systematic review and meta-analysis of the literature. Surg Endosc 29:334–348
Valls FV, Bassany EE, Jiménez-Gómez LM, Chavarría JR, Carrasco MA (2014) Robotic transanal endoscopic microsurgery in benign rectal tumour. J Robotic Surg 8:277–280
Jayne D (2015) MRC/EME ROLARR trial; the first results. In: 23rd international congress of the European association for endoscopic surgery (E.A.E.S.), Bucharest June 3–June 6
Jiang A, Ataollahi A, Althoefer K, Dasgupta P, Nanayakkara T (2012) A variable stiffness joint by granular jamming. In: ASME 2012 international design engineering technical conferences and computers and information in engineering conference, pp 267–275
Jiang A, Xynogalas G, Dasgupta P, Althoefer K, Nanayakkara T (2012) Design of a variable stiffness flexible manipulator with composite granular jamming and membrane coupling. In: IEEE/RSJ international conference on intelligent robots and systems (IROS), pp 2922–2927
Stilli A, Wurdemann HA, Althoefer K (2014) Shrinkable, stiffness-controllable soft manipulator based on a bio-inspired antagonistic actuation principle. In: IEEE/RSJ international conference on intelligent robots and systems (IROS), pp 2476–2481
Degani A, Choset H, Wolf A, Zenati MA (2006) Highly articulated robotic probe for minimally invasive surgery. In: IEEE international conference on robotics and automation proceedings, pp 4167–4172
Wei W, Kai X, Simaan N (2006) A compact two-armed slave manipulator for minimally invasive surgery of the throat. In: IEEE/RAS-EMBS international conference on biomedical robotics and biomechatronics, pp 769–774
Bajo A, Dharamsi LM, Netterville JL, Garrett CG, Simaan N (2013) Robotic-assisted micro-surgery of the throat: the trans-nasal approach. In: IEEE international conference on robotics and automation (ICRA), pp 232–238
Burgner J, Swaney PJ, Lathrop RA, Weaver KD, Webster RJ (2013) Debulking from within: a robotic steerable cannula for intracerebral hemorrhage evacuation. IEEE Trans Biomed Eng 60(9):2567–2575
Ho M, McMillan AB, Simard JM, Gullapalli R, Desai JP (2012) Toward a meso-scale SMA-actuated MRI-compatible neurosurgical robot. IEEE Trans Robot 28(1):213–222
Bajo A, Goldman RE, Long W, Fowler D, Simaan N (2012) Integration and preliminary evaluation of an insertable robotic effectors platform for single port access surgery. In: IEEE international conference on robotics and automation (ICRA), pp 3381–3387
Shang J, Noonan DP, Payne C, Clark J, Sodergren MH, Darzi A, Yang GZ (2011) An articulated universal joint based flexible access robot for minimally invasive surgery. In: IEEE international conference robotics and automation (ICRA), pp 1147–1152
Bardaro SJ, Swanstrom LL (2006) Develeopment of advanced endoscopes for natural orifice translumenal endoscopic surgery (NOTES). Minim Invasive Ther 15(6):378–383
Phee SJ, Ho KY, Lomanto D, Low SC, Huynh VA, Kencana AP et al (2010) Natural orifice transgastric endoscopic wedge hepatic resection in an experimental model using an intuitively controlled master and slave translumenal endoscopic robot (MASTER). Surg Endoscopy 24:2293–2298
Loeve A, Breedveld P, Dankelman J (2010). Scopes too flexible…and too stiff. Pulse, IEEE 1(3):26–41
Mahvash M, Dupont PE (2011) Stiffness control of surgical continuum manipulators. IEEE Trans Robot 27(2):334–345
Goldman RE, Bajo A, Simaan N (2014) Compliant motion control for multisegment continuum robots with actuation force sensing. IEEE Trans Robot 30(4):890–902
Fras J, Czarnowski J, Macias M, Glowka J, Cianchetti M, Menciassi A (2015) New STIFF-FLOP module construction idea for improved actuation and sensing. In: IEEE international conference on robotics and automation, 2015 Seattle
Małota Z, Nawrat Z, Sadowski W (2014) Minimally invasive surgery simulation. Eng Biomater 126(17):2–11
Miller K, Clavel R (1992) The lagrange-based model of delta-4 robot dynamics. Robotersysteme 8:49–54
Acknowledgments
The research leading to these results has received funding from the European Commission’s Seventh Framework Programme under Grant Agreement 287728 in the framework of EU Project STIFF-FLOP. The views expressed here are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. We would like to acknowledge Prof. Andreas Melzer and Sir Alfred Cuschieri for their hospitality in Dundee and their commendable suggestions. We would also like to acknowledge Helen McLeod for her support during test preparation and surgical procedures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Alberto Arezzo, Yoav Mintz, Marco Ettore Allaix, Giada Gerboni, Margherita Brancadoro, Matteo Cianchetti, Arianna Menciassi, Helge Wurdemann, Yohan Noh, Jan Fras, Jakob Glowka, Zbigniew Nawrat, Gavin Cassidy, Rich Walker, Simone Arolfo, Marco Bonino, Mario Morino, and Kaspar Althoefer have no conflicts of interest or financial ties to disclose.
Ethical approval
The study was carried out to appropriate ethical standards.
Informed consent
Informed consent was not required for this type of study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
The video shows the first cadaveric series of Total Mesorectal Excision (TME) using a soft and flexible robotic arm for optic vision in a cadaver model. (MP4 1035811 kb)
Rights and permissions
About this article
Cite this article
Arezzo, A., Mintz, Y., Allaix, M.E. et al. Total mesorectal excision using a soft and flexible robotic arm: a feasibility study in cadaver models. Surg Endosc 31, 264–273 (2017). https://doi.org/10.1007/s00464-016-4967-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-4967-x