Abstract
Background
The avoidance of postoperative chronic pain is of the foremost importance and has a deep impact on patient satisfaction. The objective of this study is to evaluate the selective transabdominal preperitoneal laparoscopic neurectomy for treatment of refractory inguinodynia.
Methods
Prospective study in a University Hernia Center included 16 consecutive patients with chronic pain. Primary endpoint was pain control (measured by appropriate questionnaire and need of analgesics). Secondary endpoint was surgical morbidity. Follow-up was 2 years (range 12 months–4 years).
Results
The mean operating time was 52 (range 36–68) minutes, and there were no intraoperative complications. All patients had histologic confirmation of neurectomy. Anatomical variation was found in ten patients (62.5 %), being a common trunk ilioinguinal/iliohypogastric nerve the most frequent (nine patients, 56.25 %). One patient developed hypoesthesia in the territory of the femorocutaneous nerve by nerve injury. Reoperation was performed 6 months afterward to complete ilioinguinal nerve neurectomy. Neuropathic pain medications were continued by five patients. Pain was completely eliminated in 11 (68.75 %).
Conclusions
Management of patients with neural groin pain should be done in a multidisciplinary unit. Selective neurectomy by a transabdominal preperitoneal laparoscopic approach is a safe and highly effective option in selected patients for the treatment of refractory postoperative chronic pain. Careful anatomical planning is essential to avoid inadvertent injuries and more suffering to these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Refractory neural chronic groin pain is an especially troublesome complication of many surgeries, including those performed by general surgeons (hernia repairs), gynecologists (oncological operations, hysterectomies, and oophorectomies), and urologists (renal, urethral, and bladder procedures). Most often, these patients are bounced between many specialists who are unsure of how to alleviate these patients’ discomfort. The postoperative chronic pain (PCP) is an underestimated medical problem. Reported frequency of PCP ranges from 10 to 54 % of patients, implying a severe impact on quality of life [1–3]. At present, a consensus for the management of chronic groin pain after surgery of the lower abdomen is lacking. When conservative management fails to provide relief, the most commonly accepted surgical option is that of neurectomy. Selective or triple neurectomy has been successful in several studies [4–6].
Surgical management via neurectomy was first described by Stulz and Pfeiffer [7]. The standard approach to neurectomy is an anterior open approach through the inguinal region. As an alternative, the laparoscopic retroperitoneal (RL) and transabdominal approaches have been suggested by some authors (TL) [8–10].
The objective of our prospective study is to investigate the efficacy of transabdominal preperitoneal laparoscopy for chronic postoperative groin pain in our multidisciplinary unit. We also compare our results with those retrieved from a literature review.
Materials and methods
Patients
The study was planned as a prospective single-center trial to evaluate the laparoscopic treatment in patients with refractory chronic groin pain. Between January 2012 and December 2014, 16 consecutive patients underwent laparoscopic surgery for PCP. Diagnosis was established at a specialized abdominal wall unit by personal history and physical examination; computed tomography was performed to discard possible meshoma and/or occult recurrence. Electromyography (EMG) and regional nerve blockade were used to complete diagnosis. Data were collected prospectively in a database. The study was approved by the University of San Antonio, School of Medicine, and by the Ethics Committee of the La Vega hospital. All the patients were given detailed information on the operation in accordance with good clinical practice guidelines and gave their informed consent.
Inclusion and exclusion criteria
Inclusion criteria were: (1) chronic groin pain (present for a minimum of 6 months) due to suspected nerve injury, (2) no prominent comorbidity (American Society of Anesthesiologists, ASA score: ASA I–III). Chronic pain was defined to be of neuropathic type by means of Bouhassira DN4 questionnaire (score ≥ 4) [11].
Exclusion criteria were: (1) comorbidity with ASA score > III, (2) recurrent inguinal hernia or meshoma diagnosed by tomography, (3) non-neuropathic pain syndromes or unrelated to prior surgical intervention, (4) primary orchialgia, (5) current malignant diseases, (6) proven mental illness or other circumstances that might compromise the patient’s cooperation, (7) refusal to give informed consent.
Surgical technique
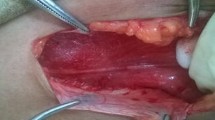
All operations were performed under general anesthesia with patients in the lateral decubitus position to allow gravity to assist with mobilization of the peritoneal viscera to the contralateral side and supported with a beanbag and axillary roll. A urinary catheter was placed for bladder decompression, and cefazolin sodium was administered for prophylaxis. The table was flexed to open the space between the iliac crest and costal margin. Pneumoperitoneum was created using a Veress needle in a subcostal space, a 10-mm trocar for the telescope was inserted, and two other 5-mm trocars were inserted under direct vision in the mid-axillary line, the inferior separate 2 cm to the spina iliaca anterior superior (Fig. 1). Peritoneum was mobilized to access the retroperitoneum. The retroperitoneal fat pad was dissected medially to expose the quadratus lumborum and psoas muscles using laparoscopic cautery. The genitofemoral nerve was visualized along the ventral surface of the psoas muscle. Separate genital and femoral trunks are often noted. Lateral femorocutaneous nerve was identified lateral to the psoas below the iliac crest. Ilioinguinal and iliohypogastric nerves were identified as a common trunk overlying the quadratus lumborum and on the ventral surface of the posterior aponeurosis of the transversus abdominis. This nerve bifurcated prior to entering the abdominal wall, near to the superior iliac spine. The subcostal nerve was identified at the T12 costal margin. Once all structures were defined, selective neurectomy was performed over the quadratus muscle or psoas muscle (Figs. 2, 3, 4). All resected nerve specimens were sent for histologic confirmation. Postoperative activity was unrestricted.
Bifurcation of the conjoined ilioinguinal and iliohypogastric at the entrance of the nerve into the muscular layers of the abdominal wall. The common trunk of the nerve should be followed distally until the branching point is reached, and the ilioinguinal and iliohypogastric nerve branches can be distinguished
Main outcome measurements
All patients were included in a follow-up program and were requested to attend a specific hernia consultation after 1, 6, and 12 months.
The primary endpoint was pain assessed by a specific questionnaire (good or pain free; moderate or some pain; poor or no effect), need of analgesics. Level of activity and disability were documented. Secondary endpoint was morbidity (hematomas, wound infection, ileus defined as no bowel movement after 24 h, urinary retention, intestinal obstructions, rejections, and readmissions). The follow-up averaged 2 years (range 12 months–4 years) and was complete in 100 % of the patients.
Statistical analysis
Values were expressed as a mean ± SD for continuous variables and as a number (%) for categorical variables. Descriptive statistics for quantitative variables and frequencies with percentages were calculated. Normal distribution of the data was tested before performing statistical analysis. Comparisons were made with ANOVA test for continuous variables and Fisher’s exact test for quantitative variables. The p value was used as the criterion for significance at p < 0.05. Data were analyzed using SPSS software package for Windows (SPSS Inc., version 18.0, Chicago, IL, USA).
Results
Patient’s mean age was 48 years (range 41–55 years). Thirteen patients had been operated for hernia repair (tension-free Lichtenstein, eight; Rutkow–Robbins, five), two patients were operated for laparoscopic apendicectomy, and one patient for a Spigelian hernioplasty.
Pharmacologic pain regimens included continuous use of narcotics in ten patients, intermittent use of narcotics in four patients, and non-opioid analgesics in two patients (Table 1).
The mean operating time was 52 (range 36–68) minutes. No intraoperative complications were registered. There were no conversions to open surgery. Operative procedures included combined IH-Ii neurectomy in nine patients, selective ilioinguinal neurectomy in five patients, and triple neurectomy in two patients. Normal anatomy was present in six patients, while variants were found in ten patients (62.5 %): a common trunk of the IIN and IHN in nine patients, and separate trunks of the GFN in one patient. All the patients were discharged within the first 24 h after operation, and all returned to their normal activities. All resected nerve specimens were normal on histologic examination (Table 2).
The neurectomy was confirmed by postoperative dermatomal mapping at 1 month. In the immediate postoperative, one patient developed hypoesthesia in the territory of the femorocutaneous nerve. Electromyography and nerve block selectively supported the clinical diagnosis of the femorocutaneous nerve injury. At 6 months, a reoperation was scheduled to complete the ilioinguinal nerve neurectomy. Mean duration of follow-up was 14 months (range 12–48 months). Neuropathic pain medications were used preoperatively by all patients, continued by five patients, and eliminated completely in 11 patients.
Literature analysis
Laparoscopic retroperitoneal neurectomy for treatment of refractory inguinodynia has been reported in five short series. We included in our review those with ≥3 cases (Table 3). Literature review shows that RL approach is associated with less morbidity with no significant differences in efficacy to relieve pain (68.75 % TL vs. 78.68 RL, p = 0.877). Our review shows that there are significant differences for pain relief in favor of the triple neurectomy (96.55 vs. 70.68 %, p = 0.003).
Discussion
Chronic pain is now considered one of the most serious complications after inguinal surgery. Its incidence may exceed 30 % [12]. Interest in this topic is demonstrated by recent publications advocating to reach a consensus on its management. Multiple options have been attempted to treat PCP. Neurectomy is advised when lasting pain control cannot be achieved [13–17]. Two questions must by consider on this surgery: Which is the safest approach? And what technique offers best results, triple or selective neurectomy?
Which is the safest approach?
Open inguinal approach is still considered as the standard. This approach allows removing the mesh if required [18]. The disadvantage is that it operates over a scar, and so can be difficult to perform, even for very experienced surgeons [19]. The overall morbidity rate is 10 % (ischemic orchitis with atrophy 4 %, testis impinged in the groin 4 %, inguinal hernia recurrence 7 %, etc.). No identifications of the nerves have been reported for the 26–76 % of the cases, and no improving in pain control in 25 % [18]. To try to improve these results, Krähenbühl et al. [20] described the RL approach. Since then have published four other studies, using this approach. We summarize the results of these studies in Table 3 [21–24]. This approach avoids dissection on a previously manipulated field and provides the known advantages of minimally invasive technique. The most common morbidity of this option is diaphragm perforation and retroperitoneal hematoma [22, 24].
In 2013, Mahan et al. [25] published in one case, the TL approach, similar to that used in this series. This option adds some other features to the classic advantages: (1) a simple and traditional access based in intraperitoneal references, (2) a better field of vision and work, providing greater security in identifying the lumbar plexus, and (3) a low risk of inadvertent injury because of a limited field of work or poor eyesight (Table 4) [26].
Our study shows that low morbidity of the RL approach is associated with no significant differences in efficacy of pain relief. For us, it stands for the most comfortable and safe approach for any general surgeon. However, nerve damage can be a concern in the event of an anatomical variation. Therefore, we must advise for a complete identification of the lumbar plexus prior to nerve section. Given the high rate of anatomical variations (>60 %) [23, 24], we recommend a regional anatomical training in the body (cadaver) to any surgeon before getting started in this technique (Fig. 5). The main disadvantage of the laparoscopic approach is the need for adequate training. Song et al. [25] considered ten neurectomies as the limit of the learning curve to guarantee a safe technique.
Which technique offers best results, triple or selective neurectomy?
Many authors recommend the triple neurectomy as a safer option, believing that any residual nerve branch could still transfer the stimulus doloroso [19, 22–27, 29]. However, this technique means a greater aggression and a larger area of swelling on the anterior thigh and genitals. Faced with this attitude, current trends encourage a selective neurectomy of the injured nerve [20, 22, 28].
This attitude requires a correct clinical mapping, well-targeted imaging tests to exclude other processes, EMG, and anesthetic blocks. The laparoscopic approach may be a minimally invasive alternative to these complex and anxious patients. Our percentage of <70 % success can be explained by the participation; to a lesser extent, other factors involved in the genesis of pain, which cannot be controlled with selective neurectomy laparoscopy: fixation material, damage to the periosteum pubic tubercle, folded or wrinkled mesh, orchialgia (which requires resection of the paravasal nerves), severe fibrosis, devitalized or necrotic tissue, sutures, etc. Furthermore, our review shows that there are significant differences in the capability to eliminate pain in favor of triple neurectomy, a fact that could be explained by the wide variability in the branching pattern of nerves, their interrelations, and overlapping areas of innervation. Lee and Dellon believe that the medical history and physical examination should be sufficient to differentiate the affected nerve in patients with chronic groin pain [9]. We propose a complete diagnosis with an EMG and nerve block, and a trial of medical treatment for at least 6 months. If no satisfactory control of pain is achieved after this conservative approach, a transabdominal preperitoneal laparoscopic neurectomy on an outpatient basis can be a safe and successful option. A new problem to consider is the abdominal wall bulging after iliohypogastric and ilioinguinal motor denervation and the emergence of pseudohernia. In our experience, pseudohernia has no clinical significance. However, patients should be informed of this potential morbidity and should be followed over time to assess their quality of life.
Conclusions
The management of patients with neural groin pain should be done in a specialized multidisciplinary unit. Selective neurectomy performed through a transabdominal preperitoneal laparoscopy is a safe and highly effective option in selected patients with refractory postoperative neuropathic pain. Anatomical knowledge and surgical training and expertise are essential to avoid inadvertent injuries that could potentially add more suffering to these patients.
References
Valvekens E, Nijs Y, Miserez M (2015) Long-term outcome of surgical treatment of chronic postoperative groin pain: a word of caution. Hernia 19(4):587–594
Loos MJ, Roumen RM, Scheltinga MR (2007) Chronic sequelae of common elective groin hernia repair. Hernia 11:169–173
Bjurstrom MF, Nicol AL, Amid PK, Chen DC (2014) Pain control following inguinal herniorrhaphy: current perspectives. J Pain Res 7:277–290
Aasvang E, Kehlet H (2009) The effect of mesh removal and selective neurectomy on persistent postherniotomy pain. Ann Surg 249:327–334
Loos MJ, Scheltinga MR, Roumen RM (2008) Surgical management of inguinal neuralgia after a low transverse pfannenstiel incision. Ann Surg 248:880–885
Ducic I, West J, Maxted W (2008) Management of chronic postoperative groin pain. Ann Plast Surg 60:294–298
Stulz P, Pfeiffer KM (1982) Peripheral nerve injuries resulting from common surgical procedures in the lower portion of the abdomen. Arch Surg 117(3):324–327
Madura JA, Madura JA II, Copper CM, Worth RM (2005) Inguinal neurectomy for inguinal nerve entrapment: an experience with 100 patients. Am J Surg 189:283–287
Lee CH, Dellon AL (2000) Surgical management of groin pain of neural origin. J Am Coll Surg 191:137–142
Kim DH, Murovic JA, Tiel RL, Kline DG (2005) Surgical management of 33 ilioinguinal and iliohypogastric neuralgias at Louisiana State University Health Sciences Center. Neuro-surgery 56:1013–1020
Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J et al (2005) Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 114:29–36
Keller JE, Stefanidis D, Dolce CJ, Iannitti DA, Kercher KW, Heniford BT (2008) Combined open and laparoscopic approach to chronic pain after inguinal hernia repair. Am Surg 74(8):695–701
Starling JR, Harms BA, Schroeder ME, Eichmin PL (1987) Diagnosis and treatment of genitofemoral and ilioinguinal entrapment neuralgia. Surgery 102:581–586
Lange JFM, Kaufmann R, Wijsmuller AR, Pierie JPEN, Ploeg RJ, Chen DC et al (2015) An international consensus algorithm for management of chronic postoperative inguinal pain. Hernia 19:33–43
Werner MU (2014) Management of persistent postsurgical inguinal pain. Langenbecks Arch Surg 399:559–569
Holloran-Schwartz MB (2014) Surgical evaluation and treatment of the patient with chronic pelvic pain. Obstet Gynecol Clin N Am 41:357–369
Chen DC, Amid PK (2014) Prevention of inguinodynia: the need for continuous refinement and quality improvement in inguinal hernia repair. World J Surg 38:2571–2573
Bischoff JM, Enghuus C, Werner MU, Kehlet H (2013) Long-term follow-up alter mesh removal and selective neurectomy for persistent inguinal postherniorrhaphy pain. Hernia 17:339–345
Campanelli G, Bertocchi V, Cavalli M, Bombini G, Biondi A, Tentorio T et al (2013) Surgical treatment of chronic pain alter inguinal hernia repair. Hernia 17:347–353
Krähenbühl L, Strifflerler H, Baer HU, Büchler MW (1997) Retroperitoneal endoscopic neurectomy for nerve entrapment after hernia repair. Br J Surg 84:216–219
Muto CM, Pedana N, Scarpelli S, Galardo R, Guida G, Schiavone V (2005) Inguinal neurectomy for nerve entrapment alter open/laparoscopic hernia repair using retroperitoneal endoscopic approach. Surg Endosc 19:974–976
Giger U, Wente MN, Büchler MW, Krähenbühl S, Lerut J, Krähenbühl L (2009) Endoscopic retroperitoneal neurectomy for chronic pain after groin surgery. Br J Surg 96(9):1076–1081
Emeksiz S, Ozden H, Guyen G (2013) Effects of variable courses of inguinal nerves on pain in patients undergoing lichtenstein repair for inguinal hernia: preliminary results. Acta Chir Belg 113:196–202
Klaassen Z, Marshall E, Tubbs RS, Louis RG, Wartmann CT, Loukas M (2011) Anatomy of the ilioinguinal and iliohypogastric nerves with observations of their spinal nerve contributions. Clin Anat 24:454–461
Song JW, Wolf JS, McGillicuddy JE, Bhangoo S, Yang LJ (2011) Laparoscopic triple neurectomy for intractable groin pain: technical of 3 cases. Neurosurgery 68:339–346
Chen DC, Hiatt JR, Amid PK (2013) Operative management of refractory neuropathic inguinodynia by a laparoscopic retroperitoneal approach. JAMA Surg 148(10):962–967
Mahan MA, Kader AK, Brown JM (2014) Robot-assisted triple neurectomy for iatrogenic inguinal pain: a technical note. Acta Neurochir (Wien) 156(1):171–175
Moreno-Egea A, Borras E (2014) Selective ambulatory transabdominal retroperitoneal laparoscopic neurectomy to treat refractory neuropathic groin pain. Rev Hispanoam Hernia 02:67–71
Amid PK (2002) A 1-stage surgical treatment for postherniorrhaphy neuropathic pain: triple neurectomy and proximal end implantation without mobilization of the cord. Arch Surg 137:100–104
Acknowledgments
The author is grateful for the San Antonio University School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Moreno-Egea has no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Moreno-Egea, A. Surgical management of postoperative chronic inguinodynia by laparoscopic transabdominal preperitoneal approach. Surg Endosc 30, 5222–5227 (2016). https://doi.org/10.1007/s00464-016-4867-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-4867-0