Abstract
Background
Randomized trials show similar outcomes after open surgery and laparoscopy for colon cancer, and confirmation of outcomes after implementation in routine practice is important. While some studies have reported long-term outcomes after laparoscopic surgery from single institutions, data from large patient cohorts are sparse. We investigated short- and long-term outcomes of laparoscopic and open surgery for treating colon cancer in a large national cohort.
Methods
We retrieved data from the Norwegian Colorectal Cancer Registry for all colon cancer resections performed in 2007–2010. Five-year relative survival rates following laparoscopic and open surgeries were calculated, including excess mortality rates associated with potential predictors of death.
Results
Among 8707 patients with colon cancer that underwent major resections, 16 % and 36 % received laparoscopic procedures in 2007 and 2010, respectively. Laparoscopic procedures were most common in elective surgeries for treating stages I–III, right colon, or sigmoid tumours. The conversion rate of laparoscopic procedures was 14.5 %. Among all patients, laparoscopy provided higher 5-year relative survival rates (70 %) than open surgery (62 %) (P = 0.040), but among the largest group of patients electively treated for stages I–III disease, the approaches provided similar relative survival rates (78 vs. 81 %; P = 0.535). Excess mortality at 2 years post-surgery was lower after laparoscopy than after open surgery (excess hazard ratio, 0.7; P = 0.013), but similar between groups during the last 3 years of follow-up. Major predictors of death were stage IV disease, tumour class pN+, age > 80 years, and emergency procedures (excess hazard ratios were 5.3, 2.4, 2.1, and 2.0, respectively; P < 0.001).
Conclusion
Nationwide implementation of laparoscopic colectomy for colon cancer was safe and achieved results comparable to those from previous randomized trials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Colorectal cancer is the third most common cause of death in Western Europe and the USA, and its incidence is increasing [1]. Surgical resection remains the cornerstone of cure, and it is also employed as a palliative treatment to relieve detrimental symptoms in patients with advanced disease. Laparoscopic colectomy was first reported in 1991 [2], and it has since emerged as an alternative to open colectomy. However, laparoscopic colon resection is considered a rather complex procedure. Thus, the surgical community’s adoption of the laparoscopic approach for colorectal surgery has been slower and less widespread compared to the adoption of laparoscopic procedures for other indications.
Randomized controlled multicentre trials have shown similar long-term survival outcomes with open and laparoscopic techniques [3–6]. However, randomized trials have strict inclusion criteria, and generalizability to other populations may be limited by patient selection. Accordingly, it is important to assure that outcomes from randomized trials can be reproduced in large patient cohorts that reflect common surgical practice for treatment of this life-threatening disease [7, 8]. Presently, data on the extent that laparoscopic colon resection is used for treating cancer in routine clinical practice and the outcomes of those treatments are currently limited [9], because most data in contemporary literature were obtained from retrospective institutional audits [10–13].
In the present study, short- and long-term outcomes were evaluated in a large national cohort after laparoscopic surgery for colon cancer was introduced into routine clinical practice.
Materials and methods
This observational study was conducted according to the suggested STROBE guidelines [14].
Ethics
Data were reported and collected according to governmental regulations for the Cancer Registry of Norway. Anonymous data were provided for analyses. The study was classified as an evaluation of current practice; thus, it did not require approval of the Regional Ethics Committee.
Study population
We evaluated data recorded in the Cancer Registry of Norway (CRN) regarding patients treated with major surgical resections for primary colon cancer, between 1 January 2007 and 31 December 2010. National legislation requires that all patients diagnosed with solid tumours must be recorded in the CRN. Data on patients with colon cancer are also recorded in the Norwegian Colorectal Cancer Registry (NCCR), which is a clinical quality registry that forms part of the CRN. Hospitals nationwide record data on patient demographics, disease stage, treatments employed, and follow-up information, including recurrences. All residents of Norway have a unique, 11-digit personal number that enables complete patient follow-up. The dates of death were obtained from Statistics Norway (http://www.ssb.no) in February, 2014. The registry also made regular queries to the responsible hospitals to collect missing and follow-up data on clinical outcomes, including times and locations of recurrent disease.
Clinical management
Surgery was performed according to national guidelines, based on current surgical state-of-the art practices. The decision of whether to use an open or laparoscopic approach was based on hospital policy and the technical skills of the surgical staff. In accordance with the national standard of care, laparoscopic surgery was considered when the surgical team and department had the necessary experience, and when preoperative examinations indicated that tumour resection was technically feasible. Development of laparoscopic skills was the responsibility of the individual surgical departments, and did not occur within a national training programme. The national guidelines for elective cases recommended a preoperative work-up that included a complete bowel examination, with or without a biopsy, for malignant tumour confirmation; a measurement of serum carcinoembryonic antigen (CEA); and a computed tomography (CT) scan of the chest and abdomen. In the emergency setting, patient examinations were based on their clinical presentation and the time available before urgent surgery. Patients were followed for 5 years. Follow-up examinations included a biannual serum CEA measurement, a biannual contrast-enhanced ultrasound examination of the liver, and an annual low-dose CT scan of the chest. Any loco-regional or distant recurrences, identified according to criteria defined in national guidelines, were reported to the NCCR [15]. No data were available regarding BMI, previous abdominal surgery, specific comorbidity, or surgeon experience.
Definitions
Demographic data included gender and age. Age groups were defined as ≤ 65 years (working age), 65–79 years (retired and generally eligible for active surgical or oncological treatment of recurrences), and ≥ 80 years of age (aged patients). An anastomotic leak was defined as a leak from the anastomosis, confirmed by imaging or reoperation.
Surgery
Laparoscopic resection was defined as any resection performed laparoscopically that did not require conversion to open surgery. Conversion to open surgery was defined as a procedure that had commenced as a laparoscopic procedure, but eventually, for any reason, was converted to a laparotomy. For the present analyses, surgical procedures were categorized as right-sided resections (including the transverse colon), left non-sigmoid resections (including the left colon flexure and the descending colon), or sigmoid resections. Patients with resections of the recto-sigmoid junction that included part of the rectum were excluded.
Hospital volume and teaching status
In Norway, surgery for colon cancer is exclusively offered in public, government-owned, non-profit hospitals. The hospitals included in this study were categorized according to the annual number of colon resections for cancer; hospitals performed either < 25 or ≥ 25 annual resections (i.e. < 2 or ≥ 2 resections per month) [16, 17]. Hospitals were also classified as either teaching hospitals (i.e. those that provided a fellowship programme for subspecialist training in gastrointestinal surgery) or non-teaching hospitals (i.e. those without an approved teaching programme).
Staging and tumour characteristics
Cancer stage was classified according to the TNM classification (7th edition) [18]—which takes into account the extent of the primary tumour (T), the involvement of regional lymph nodes (N), and the presence or absence of distant metastases (M)—expressed in Arabic numeral notations. Disease stage was based on cancer staging, expressed in Roman numerals. The colon was defined as the large intestine, starting at 15 cm above the anal verge and extending to the caecum. Tumour size was categorized as either < 5 or ≥ 5 cm in diameter.
Statistical analysis
Statistical analyses were performed with IBM SPSS Statistics (IBM Corporation) version 22 and with R 3.1.0 (http://www.r-project.org). The R-package “relsurv” version 2.0-6 was employed to calculate relative survival and excess mortality [19]. Parameters were estimated with the maximum likelihood method. Data regarding demographics and clinico-pathological characteristics were analysed with descriptive statistics. The Chi-squared test was used to compare categorical variables. For dichotomous outcome variables, we performed multivariable logistic regression analyses with variable selection and evaluation of goodness of fit, according to Hosmer and Lemeshow’s purposeful variable selection method [20].
Survival up to 5 years was calculated with a relative survival analysis, in which the probability of patients with colon cancer surviving to a given time post-operatively was divided by the probability that the general population would survive to that time, given the same age, gender, and year of birth distributions. Population survival was calculated with Norwegian population lifetime tables from Statistics Norway (http://www.ssb.no). The excess mortality was defined as the additional mortality observed in the patient group compared to the mortality of the general population, given the same age, gender, and year of birth distributions [21]. The excess mortality was as a step function which was constant over each of the post-operative time intervals studied: 0–3 months, 3–12 months, 1–2 years, 2–3 years, 3–4 years, and 4–5 years. Patients that received laparoscopies were compared to those that received open surgeries by calculating excess mortality ratios, defined as the ratio of the respective excess mortality rates for each post-operative time interval. This analysis was performed on an intention-to-treat basis, in the sense that converted procedures were considered laparoscopic. In addition, we grouped patients with loco-regional disease (i.e. stages I, II, and III) into one group and compared them to patients with stage IV disease, because preoperative imaging typically can detect only gross tumour invasions beyond the colon and mesocolic fat (i.e. T4). More detailed T staging, based on CT scans, is currently unreliable.
After estimating basic excess mortality, we performed multivariable analyses with a multiplicative model for the excess hazard ratio [19]. We included factors that could potentially influence mortality as covariates. The multivariable analyses then identified which factors significantly impacted excess mortality. These analyses ensured that our estimates of excess mortality ratios were unbiased [22]. To guard against type 1 errors in multiple testing, we used a level of 0.01 for the inference in each time interval, when reporting excess mortality ratios for each time interval during the 5-year follow-up. This level of 0.01 corresponded to the use of an overall level of 0.05, with Bonferroni correction for multiple testing. All other tests were two-tailed, and the significance level was set at P < 0.050.
Results
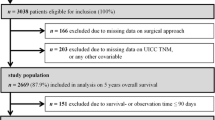
During the study period, a total of 10,648 patients were registered with a first-time diagnosis of colon cancer. Among these, 8707 patients (82 %) underwent major resections at 54 different Norwegian hospitals (Fig. 1). We excluded patients with incomplete data regarding disease stage (n = 95; 0.9 %) or surgical approach (n = 1589; 14.9 %; Supplemental Table S1). Thus, a total of 7023 patients with major colon resections were eligible for analysis. Of those, 5036 (72 %) patients had TNM stages I–III colon cancer and were treated electively.
Flowchart of study patient selection. The laparoscopic group (n = 1901) included 275 procedures that are converted to open surgery. Analysis of patients with missing data on the surgical approach (n = 1589; Supplemental Digital Content Table 1) showed no significant differences from the included group, in terms of gender (P = 0.109), emergency presentations (P = 0.082), or annual patient volume at the treating hospital (P = 0.113). However, patients excluded due to missing data had a significantly lower frequency of reported anastomotic leaks (P < 0.004) and a higher frequency of stage IV disease (P = 0.047) compared to the included cohort
Table 1 shows patient demographics and clinico-pathologic characteristics. A laparoscopic approach was attempted in 1901 operations (27.1 %), and 275 (14.5 %) of these procedures were converted to open surgery. During the study period, the proportion of laparoscopic procedures increased from 15.9 % in 2007 to 35.7 % in 2010 (P < 0.001). Among the 54 hospitals included in this study, 9 (16.7 %) performed laparoscopic colon surgery in 2007 as compared to 19 (35.2 %) in 2010. Gender and age distributions were similar between the groups treated with different surgical approaches. Laparoscopic surgery was more common in patients with right-sided and sigmoid tumour locations compared to those with left-sided tumours. Laparoscopy was more common in pT1–3 than in pT4 tumours. The proportion of patients with at least 12 harvested lymph nodes in laparoscopic resections was 78.3 %, as compared to 72.4 % in open surgery (P < 0.001).
Selection of surgical approach
Clinical and tumour-related characteristics were included in univariable and multivariable regression analyses to identify factors that might be related to the selection of the different surgical approaches (Table 2). Multivariable regression analyses showed that elective treatment was the most important factor in the choice of laparoscopy over open surgery (OR 4.1; 95 % CI 2.53–6.80). Other significant factors included a less advanced T stage (pT1–3 versus pT4; OR 1.7; 95 % CI 1.36–2.13) and smaller tumour size (< 5 versus ≥ 5 cm; OR 1.2; 95 % CI 1.05–1.35). Compared to tumours in the right colon or the sigmoid colon, tumours in the left colon were less likely to be resected with laparoscopic surgery (OR 0.5; 95 % CI 0.38–0.63). Patients treated at non-teaching hospitals were less likely to undergo laparoscopic surgery (OR 0.1; 95 % CI 0.02–0.41) when compared to patients treated at teaching hospitals.
Conversion from laparoscopy to open surgery
Among procedures that were commenced as laparoscopic, the overall conversion rate was 14.5 %. This rate remained unchanged throughout the study period. Multivariable regression analysis showed that, compared to right-sided tumours, conversion rates were higher for left-sided tumours (OR 3.8; 95 % CI 2.26–6.52) and sigmoid tumours (OR 1.8; 95 % CI 1.29–2.45). Higher conversion rates were also associated with pT4 tumours (OR 3.5; 95 % CI 2.29–5.48), acute surgery (OR 3.3; 95 % CI 1.85–6.06), and TNM stage IV (OR 2.0; 95 % CI 1.36–2.96). Gender, age, and annual patient volume at the treating hospital were not significant predictors for conversion (Supplemental Table S2).
Anastomotic leaks with different procedures
The anastomotic leak rate was significantly lower after laparoscopy (3.2 %) than after open surgery (5.3 %) (P < 0.001). This difference remained significant also when comparisons were limited to patients treated electively for stages I–III disease (3.4 % and 5.3 %, respectively; P < 0.001). Multivariable regression analysis revealed that the anastomotic leak rate was associated with gender (male vs. female: OR 1.7; 95 % CI 1.32–2.14) and surgical approach (laparoscopic vs. open surgery: OR 0.5; 95 % CI 0.38–0.72) (Table 3).
Survival
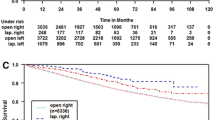
Regardless of the surgical approach, the median follow-up period was 61 (range 38–85) months. The relative survival at 5 years was 70.0 % in the laparoscopic group (n = 1901) and 62.1 % in the open surgery group (n = 5122) (P = 0.040) (Fig. 2A). When analysing patients that underwent elective resections for stages I–III disease (n = 5857), the relative survival was 77.7 % in the laparoscopic group and 80.6 % in the open group (P = 0.535) (Fig. 2B). Survival was similar within stage I, II, or III when analysed separately. In patients with stage IV disease, the relative 5-year survival was 19 % in both groups after colon resections (Fig. 2C).
Five-year relative survival rates. The relative survival is calculated as the number of patients that received either laparoscopic or open surgical resections for colon cancer between 2007 and 2010 compared to the number of age- and sex-matched individuals in the general population that survived over the same 5-year period. Five-year relative survival rate are shown for A all patients, B all patients with stages I–III disease that received elective treatments, and C all patients with stage IV disease
Excess mortality and multivariable analysis
The excess mortality ratios of laparoscopic to open surgery are shown in Table 4 and Fig. 3. Excess mortality (with P at the 0.01 level of significance) was lower in the laparoscopic group than in the open group during the first two years after surgery, and the rates were similar thereafter. However, when this analysis was limited to patients that received elective surgery for stages I–III disease, the excess mortality was similar at all time intervals of follow-up (P level at 0.01).
Excess mortality ratios for different patient groups. Excess hazard ratios are calculated for patients with colon cancer treated between 2007 and 2010. This ratio compares excess mortality rates between the two treatment groups, i.e. the excess mortality of the laparoscopic group to the excess mortality of the open surgery group. Excess hazard ratios are shown with 99 % confidence intervals for various time intervals during the 5-year follow-up. Ratios are calculated for A all patients, B patients treated electively for stage I–III disease, and C patients treated for stage IV disease
Multivariable analyses of other possible predictors of 5-year survival showed that male patients had a significantly increased risk of death compared to females, when operated electively for stages I–III tumours (Table 4). A higher age was a significant predictor of death in all stratified patient groups; this result indicated that the diagnosis and treatment of colon cancer was associated with a higher risk of death in patients ≥ 80 years compared to patients in younger age groups, regardless of surgical approach. Surgery for sigmoid tumours was associated with a significantly lower risk of death compared to resections of the right colon (excess hazard, 0.6; P < 0.001), but not resections of the left non-sigmoid colon, for stages I–III disease. Emergency procedures were associated with a twofold increase (P < 0.001) in the 5-year mortality for patients with stages I–III disease, and with a 1.6-fold increase (P < 0.001) for patients with stage IV disease. Lymph node involvement (i.e. pN+) was the strongest predictor of the 5-year risk of death; this condition increased the excess hazard by 3.1 (P < 0.001) among patients with stages I–III disease that received elective treatments.
Discussion
This study shows that nationwide implementation of laparoscopy in the surgical treatment of colon cancer has been safe, with long-term survival similar to that achieved with open surgery in the setting of common daily practice in a large national patient cohort.
Several randomized controlled trials (RCTs) [4, 23–27], systematic reviews [3], and meta-analyses [5, 6] have reported similar long-term survival rates for patients that received laparoscopic and open surgery for colon cancer resections. These studies, however, were mostly phase III trials, and they imposed strict patient inclusion criteria. Also, those operations were most likely performed by surgeons with special interest and skills in laparoscopy, and often in highly specialized hospitals. Thus, it may be difficult to generalize those results to a general population, due to selection bias. Currently, there is only sparse information available on the long-term outcomes of laparoscopic treatments for colon cancer performed in routine surgical practice. Accordingly, it is important to determine whether the outcomes from RCTs can be reproduced in large patient cohorts managed in a daily practice setting, which is comparable to a phase IV or implementation study.
To reflect this setting, we used an intention-to-treat analytical approach by grouping the patients according to preoperative information regarding disease stage (i.e. loco-regional, stages I–III disease, or stage IV disease) and by grouping converted procedures with completed laparoscopic surgeries for the analyses. Our results confirmed that laparoscopic surgery for colon cancer could be safely performed in a routine practice setting for the subgroup of patients treated electively for limited disease (i.e. stages I–III and/or tumour sizes < 5 cm). Relative survival was high within the largest subgroup of patients with less advanced disease (i.e. stages I–III, tumour size < 5 cm) that was treated electively. This selection of patients to laparoscopy complies with current recommendations regarding the safe performance of laparoscopic resection for curable colon and rectal cancer as defined by the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) [28] and the British guidelines for case selection for laparoscopic colorectal resection from the National Training Programme for Laparoscopic Colorectal Surgery [29]. At the time of introduction of laparoscopic surgery, no formal guidelines or national training programme existed in Norway. These results observed in this study were most likely due to reasonable patient selection.
Patients treated by laparoscopic surgery showed significantly better relative survival and lower excess mortality during the first 2 years of follow-up, when compared to those with open surgery. However, no significant between-group differences were observed during the later 3 years (Table 4). In fact, during the last years of follow-up, there was a slightly higher mortality rate among patients that received laparoscopic surgery than those that received open surgery. This result apparently counteracted the initial favourable results observed in the laparoscopic group (Fig. 3 A–C). However, these differences did not reach statistical significance, probably due to the fewer patients included in the analyses of the later years of follow-up. This assumption was supported by the large confidence intervals. Overall, our findings may indicate that laparoscopic surgery was associated with some beneficial effects during the first two years post-surgery; however, we lack sufficient data to elaborate on this hypothesis further. We observed no between-group differences in excess mortality among the patients with stages I–III disease that were treated electively, nor when calculating relative survival in stage I, II, or III separately. This lack of difference may be attributable to selection, because patients that received operations in an emergency setting were excluded.
This study suggested that the laparoscopic approach was rapidly and widely implemented during the study period. Most hospitals with a large annual colectomy volume (> 25 operations) and most teaching hospitals have incorporated laparoscopic colon resections as part of their routine practice. The procedure-type conversion rate did not change during the study period. Although we had no data regarding the technical skills or experience of individual surgeons, there was most likely a gradual increase in the number of patients with colon cancer selected for laparoscopy, in parallel with the increase in surgeon experience with laparoscopic surgery throughout the study period. The implementation of laparoscopic surgery is, as any new surgical technique, associated with a learning curve, and may lead to longer operation time, increased complications or a higher frequency of conversion to open surgery. This may be particularly true in the setting of low-volume hospitals. Unfortunately, the NCCR does not provide detailed data for further evaluation.
We found that tumours located in the right colon and the sigmoid were more likely to be treated with a laparoscopic approach than tumours in the left colon. This was presumably due to the infrequent occurrence and technical challenges associated with left colon tumours. The careful selection of patients that received laparoscopic resection helped make this procedure a safe surgical alternative to conventional open surgery. The data showed that laparoscopic resection was associated with a lower anastomotic leak frequency and similar relative survival compared to open surgery. However, the significantly lower frequency of anastomotic failure was not consistent with results from prior randomized studies [25, 30]. Our data were insufficient to explain this difference clearly; however, we suspect that factors known to influence favourable outcomes, such as BMI, comorbidity, previous abdominal surgery, and surgeon skills, also most likely influenced the selection of patients for laparoscopy.
Derived from a national registry, the present study cohort included patients that underwent colon resection between 2007 and 2010. The end of this study period was contemporary with the first release of long-term results for laparoscopic procedures. Thus, fewer procedures were performed at the start of national implementation, and the number of procedures may have increased towards the end of the study period, with surgeon experience and confirmation of long-term results from RCTs [4, 5, 31–34]. We included patients that underwent resection up to 2010, and we collected follow-up data for approximately 5 years.
This study had some limitations. The most conspicuous limitation was the absence of data regarding patient perioperative comorbidity and functional status, which is an inherent limitation of most national registry studies. We also lacked data on surgeon experience; however, we note that the conversion rate from laparoscopic to open surgery did not change significantly during the 4-year study period and that the conversion rate was considerably lower than that typically found in randomized trials [4, 25]. These observations suggested that surgeon experience did not substantially impact the results.
Missing data are generally acknowledged as a disadvantage and a challenge of large national registries. Missing data on surgical approach in some 15 % of the patients are most likely due to suboptimal reporting from the various hospitals, and may have the potential of introducing a selection bias. Therefore, we investigated the possibility of a selection bias regarding the group with missing data on surgical approach. Table S1 displays various characteristics with regard to missing data. Accordingly, we think that the missing of data in this regard has a very limited systematic influence on outcomes, if any. Furthermore, because we retrieved data from the national registry, we did not aim to identify causative relationships; instead, we aimed to provide a clear depiction of whether the laparoscopic approach in daily routine could achieve outcomes similar to those achieved with open surgery in a large, unselected patient cohort. The use of relative survival as the main outcome depicted mortality associated with the diagnosis and treatment of colon cancer, and thus, a comparison of these two surgical approaches from a national perspective was warranted.
In conclusion, this analysis showed that laparoscopic surgery for colon cancer was safe and feasible in routine practice with regard to short- and long-term outcomes, and thus adds support to previous results reported from randomized controlled trials.
References
International Agency for Research on Cancer, World Health Organization (2014) World Cancer Report 2014. In: Steward BWW, C.P. (ed), World Health Organization, pp 392–402
Jacobs M, Verdeja JC, Goldstein HS (1991) Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc 1:144–150
Kuhry E, Schwenk W, Gaupset R, Romild U, Bonjer J (2008) Long-term outcome of laparoscopic surgery for colorectal cancer: a cochrane systematic review of randomised controlled trials. Cancer Treat Rev 34:498–504
Colon Cancer Laparoscopic or Open Resection Study Group, Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, Lacy A, Bonjer HJ (2009) Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol 10:44–52
Di B, Li Y, Wei K, Xiao X, Shi J, Zhang Y, Yang X, Gao P, Zhang K, Yuan Y, Zhang D, Wei X, Liu S, Wang J, Wang X, Zhang Y, Cai H (2013) Laparoscopic versus open surgery for colon cancer: a meta-analysis of 5-year follow-up outcomes. Surg Oncol 22:e39–e43
Theophilus M, Platell C, Spilsbury K (2014) Long-term survival following laparoscopic and open colectomy for colon cancer: a meta-analysis of randomized controlled trials. Colorectal Dis 16:O75–O81
Lassen K, Hvarphiye A, Myrmel T (2012) Randomised trials in surgery: the burden of evidence. Rev Recent Clin Trials 7:244–248
McCulloch P, Taylor I, Sasako M, Lovett B, Griffin D (2002) Randomised trials in surgery: problems and possible solutions. BMJ 324:1448–1451
Miskovic D, Wyles SM, Carter F, Coleman MG, Hanna GB (2011) Development, validation and implementation of a monitoring tool for training in laparoscopic colorectal surgery in the English National Training Program. Surg Endosc 25:1136–1142
Petrelli NJ (2015) Do we really need another article on minimally invasive colorectal cancer surgery? J Natl Cancer Inst 107:376
Zheng Z, Jemal A, Lin CC, Hu CY, Chang GJ (2015) Comparative effectiveness of laparoscopy vs open colectomy among nonmetastatic colon cancer patients: an analysis using the National Cancer Data Base. J Natl Cancer Inst 107
Alnasser M, Schneider EB, Gearhart SL, Wick EC, Fang SH, Haider AH, Efron JE (2014) National disparities in laparoscopic colorectal procedures for colon cancer. Surg Endosc 28:49–57
Neudecker J, Bergholz R, Junghans T, Mall J, Schwenk W (2007) Laparoscopic sigmoidectomy in Germany—a standardised procedure? Langenbecks Arch Surg 392:573–579
Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M, Initiative S (2007) Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Epidemiology 18:805–835
Norwegian Gastro-Intestinal Cancer Group (2015) Nasjonalt handlingsprogram for kreft i tykk- og endetarm. Helsedirektoratet
Borowski DW, Bradburn DM, Mills SJ, Bharathan B, Wilson RG, Ratcliffe AA, Kelly SB, Northern Region Colorectal Cancer Audit Group (2010) Volume-outcome analysis of colorectal cancer-related outcomes. Br J Surg 97:1416–1430
Nedrebø BSO, Søreide K, Nesbakken A, Eriksen MT, Søreide JA, Kørner H (2013) Risk factors associated with poor lymph node harvest after colon cancer surgery in a national cohort. Colorectal Dis 15:E301–E308
Sobin LH, Gospodarowicz MK, Wittekind C (2009) The TNM classification of malignant tumours (7th edn). Wiley-Blackwell, New York, p 336
Pohar M, Stare J (2006) Relative survival analysis in R. Comput Methods Progr Biomed 81:272–278
Lemeshow S, Hosmer DW Jr (1982) A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol 115:92–106
Dickman PW, Sloggett A, Hills M, Hakulinen T (2004) Regression models for relative survival. Stat Med 23:51–64
Perme MP, Stare J, Esteve J (2012) On estimation in relative survival. Biometrics 68:113–120
Braga M, Frasson M, Zuliani W, Vignali A, Pecorelli N, Di Carlo V (2010) Randomized clinical trial of laparoscopic versus open left colonic resection. Br J Surg 97:1180–1186
Lacy AM, Delgado S, Castells A, Prins HA, Arroyo V, Ibarzabal A, Pique JM (2008) The long-term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann Surg 248:1–7
Fleshman J, Sargent DJ, Green E, Anvari M, Stryker SJ, Beart RW Jr, Hellinger M, Flanagan R Jr, Peters W, Nelson H, Clinical Outcomes of Surgical Therapy Study Group (2007) Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg 246:655–662 (discussion 662–654)
Liang JT, Huang KC, Lai HS, Lee PH, Jeng YM (2007) Oncologic results of laparoscopic versus conventional open surgery for stage II or III left-sided colon cancers: a randomized controlled trial. Ann Surg Oncol 14:109–117
Allaix ME, Giraudo G, Mistrangelo M, Arezzo A, Morino M (2015) Laparoscopic versus open resection for colon cancer: 10-year outcomes of a prospective clinical trial. Surg Endosc 29:916–924
Society of American Gastrointestinal and Endoscopic Surgeons (2015) Guidelines for laparoscopic resection of curable colon and rectal cancer. http://www.sages.org/publications/guidelines/guidelines-for-laparoscopic-resection-of-curable-colon-and-rectal-cancer/. Accessed on 18 Dec 18 2015
NHS Lapco Guidelines for case selection for laparoscopic colorectal resection. http://lapco.nhs.uk/img/MDTCaseSelection.pdf. Accessed on 18 Dec 18 2015
Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, Lacy AM, Group COcLoORS (2005) Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol 6:477–484
Stucky CC, Pockaj BA, Novotny PJ, Sloan JA, Sargent DJ, O’Connell MJ, Beart RW, Skibber JM, Nelson H, Weeks JC (2011) Long-term follow-up and individual item analysis of quality of life assessments related to laparoscopic-assisted colectomy in the COST trial 93-46-53 (INT 0146). Ann Surg Oncol 18:2422–2431
Ohtani H, Tamamori Y, Arimoto Y, Nishiguchi Y, Maeda K, Hirakawa K (2012) A meta-analysis of the short- and long-term results of randomized controlled trials that compared laparoscopy-assisted and open colectomy for colon cancer. J Cancer 3:49–57
Bagshaw PF, Allardyce RA, Frampton CM, Frizelle FA, Hewett PJ, McMurrick PJ, Rieger NA, Smith JS, Solomon MJ, Stevenson AR, Australasian Laparoscopic Colon Cancer Study Group (2012) Long-term outcomes of the australasian randomized clinical trial comparing laparoscopic and conventional open surgical treatments for colon cancer: the Australasian Laparoscopic Colon Cancer Study trial. Ann Surg 256:915–919
Huscher CG, Bretagnol F, Corcione F (2015) Laparoscopic colorectal cancer resection in high-volume surgical centers: long-term outcomes from the LAPCOLON Group trial. World J Surg
Acknowledgments
This study used data from the Cancer Registry of Norway. The interpretation and reporting of these data are the sole responsibility of the authors, and no endorsement by the Cancer Registry of Norway is intended nor should be inferred. The authors are grateful for financial support provided by the Folke Hermansen Fund for Cancer Research at Stavanger University Hospital, and for valuable advice and comments from the members of the Norwegian Colorectal Cancer Group.
Disclosures
This study was funded by Folke Hermansen’s Fund for Cancer Research at Stavanger University Hospital, Grant #424507. The funding institution did not have any influence on the process of analysis of data, interpretation of results or preparation of the present manuscript. None of the authors (Drs. K. Stormark, K. Søreide, J.A. Søreide, J.T. Kvaløy, F. Pfeffer, M.T. Eriksen, B.S. Nedrebø, and H. Kørner) have any conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the Norwegian Colorectal Cancer Group.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stormark, K., Søreide, K., Søreide, J.A. et al. Nationwide implementation of laparoscopic surgery for colon cancer: short-term outcomes and long-term survival in a population-based cohort. Surg Endosc 30, 4853–4864 (2016). https://doi.org/10.1007/s00464-016-4819-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-4819-8