Abstract
Introduction
Anastomotic leakage (AL) is a major complication of colorectal surgery. The leakage is classified as grade B when the patient’s clinical condition requires an active therapeutic intervention but does not require further surgery. The management of grade B AL commonly includes administration of antibiotics and/or the placement of a pelvic drainage performed under radiological guidance or transanal drain. The objective of this study was to evaluate the feasibility and the efficacy of endoscopic transanastomotic drainage using double-pigtail stents (DPSs) in the management of grade B AL in colorectal surgery.
Patients and methods
Between September 2011 and December 2014, 650 patients underwent a colorectal procedure in our university hospital; 8.7 % presented with AL, including 42.8 % with grade B. Fourteen patients required endoscopic management and constituted the study population. The study’s primary objective was to assess the feasibility and efficacy of DPS placement for the treatment of grade B AL after colorectal surgery. The secondary endpoints were the requirement for radiological drainage, the DPS placement failure rate, the rate of stoma closure and, lastly, feasibility of chemotherapy (if indicated).
Results
DPS placement was feasible in 92.8 % of the 14 patients (n = 13). The overall success rate for endoscopic management was 78.5 % (n = 11). The median length of hospitalization after DPS placement was 5 days (3–17). The average duration of drainage through a DPS was 62 days (28–181). Five patients (35.7 %) also underwent drainage with radiological guidance. Of the 10 patients with stoma, closure occurred in 80 %. All patients that required adjuvant chemotherapy were able to receive it.
Conclusion
The treatment of AL requires multidisciplinary collaboration to save the anastomosis. DPS placement under endoscopic control is associated with AL healing, good clinical tolerance and the ability to undergo chemotherapy and is an alternative to repeat laparotomy when radiological drainage is unfeasible or inefficient.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Anastomotic leakage (AL) is a major complication of colorectal surgery. The incidence of AL varies from 2.4 to 12 %; this broad range is partly due to the differing definitions of AL used in the literature [1–4]. Anastomotic leakage has immediate short-term consequences in terms of morbidity, mortality and hospitalization duration [5–7]. It also has long-term consequences through alteration of the functional outcome [8]; this may lead to the creation of a definitive, terminal stoma or the non-closure of a defunctioning stoma [9] and reduction in survival [10].
In the absence of organ failure, the main therapeutic goal in cases of AL is to save the anastomosis. Anastomotic leakage is classified as grade B when the patient’s clinical condition requires an active therapeutic intervention that can be managed without further surgery [4]. The management of grade B AL commonly includes (for colorectal and coloanal anastomosis) the administration of antibiotics and/or the placement of a pelvic drain under radiological guidance or (for coloanal anastomosis) a transanal drain [4]. In some cases, the intended pelvic or transanal drainage is unfeasible or inefficient; further surgery is then the very last option in patients without organ failure and is often delayed if the patient’s condition allows.
The use of a double-pigtail stent (DPS) has been described in the treatment of pancreatic pseudocysts [11, 12] and gastric fistula after sleeve gastrectomy [13]. This technique may thus be of value in the management of colorectal fistula/abscess. It could be proposed as an alternative to repeat laparotomy or when radiological drainage is unfeasible or inefficient, in order to avoid anastomosis resection and decrease the length of hospital stay.
The objective of the present pilot study was to evaluate the feasibility and efficacy of endoscopic-guided drainage using a DPS in the management of grade B AL in colorectal surgery.
Patients and methods
Population and inclusion criteria
From September 2011 to December 2014, 650 patients underwent a colorectal procedure (elective and emergency surgery, benign and malignant disease) in our university hospital. Fifty-six of the patients demonstrated AL (8.7 %, including 6 grade A cases (10.7 %) and 24 grade B cases (42.8 %)). Fourteen of the latter required endoscopic management and thus constituted the study population. Lastly, there were 26 cases (46.4 %) of grade C AL (Fig. 1). We excluded patients with grade B AL who had been successfully treated with radiological drainage and patients with grade A or C AL.
Study design
We performed a retrospective, single-centre study. The data were extracted from a database that has been prospectively maintained since November 2003.
Study criteria
-
Patient-related criteria: age, gender, body mass index (BMI), comorbidities, signs leading to the diagnosis of AL
-
Criteria related to the initial surgery: surgical indications and procedures (including the type of anastomosis).
-
Criteria related to endoscopy and DPS placement:
-
The feasibility of DPS placement, defined as the proportion of successful placements.
-
The success rate for endoscopic management, defined as a healing of AL (with no abscesses or fistula) on a contrast-enhanced CT scan (Fig. 2).
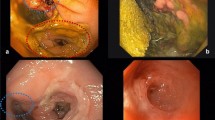
Fig. 2 DPS placement procedures. A A contrast-enhanced CT scan revealed AL (leak of contrast at the anastomosis or the rectal stump); B the orifice of the fistula was dilated with a 0.035-in. guide wire; C an X-ray view at the end of the procedure, showing the transanastomotic DPS; D a contrast-enhanced CT scan showing the disappearance of the AL and the presence of the transanastomotic DPS; E an endoscopic view of the transanastomotic DPS; F an X-ray view after treatment with a DPS, showing the absence of AL
-
The number of endoscopic procedures, the number of DPS implanted, the management of associated radiological drains, the management of DPS placement failures and long-term outcomes (including the stoma closure rate in patients with stoma and the chemotherapy feasibility rate in patients requiring chemotherapy).
-
Endpoints
The study’s primary endpoint was the DPS placement rate and the success rate for the effective treatment of grade B AL after colorectal surgery. The secondary endpoints were the description of the management of associated radiological drains, the management of DPS placement failure, the stoma closure rate and chemotherapy feasibility. Data relative to patients treated with radiological drains were also reported.
Definition of colorectal AL
The definition of colorectal AL has been clarified by the International Study Group of Rectal Cancer (ISGRC): AL includes all anastomotic defects (including suture and staple lines of the neorectal reservoirs) leading to communication between the intra- and extraluminal compartments [4]. The ISGRC classified AL into three grades. Grade A AL corresponds to “radiological leakage” in the absence of clinical symptoms or abnormal laboratory tests. The patient is clinically well, and no active therapy is necessary. Anastomotic leakage is classified as grade B when the patient’ clinical condition requires an active, therapeutic intervention other than surgery (e.g. administration of antibiotics, radiological placement of a pelvic drain and/or transanal lavage). Lastly, patients with grade C AL require repeat laparotomy (Fig. 2) [4].
Management of grade B AL and indications for an endoscopic procedure
All cases of grade B AL were discussed in a multidisciplinary team meeting by colorectal surgeons, specialist radiologists and endoscopists. All patients had received a broad spectrum of intravenous antibiotics against Gram-negative and anaerobic organisms, which was subsequently modified as a function of the bacteriological test results. Whenever possible, we placed a suction drain (with radiological guidance) or a transanastomotic drain (under general anaesthesia, for coloanal anastomosis). The drain was irrigated in all cases (10 ml, three times daily), and all patients were checked with a CT scan 7 days after drain placement. If the patient no longer displayed clinical signs, abnormal laboratory results or collections in the CT scan, the drain was removed.
When the radiological drainage was not feasible or was ineffective because of persistent collection or the reappearance of collection after the drain was removed, endoscopic drainage was considered during a weekly multidisciplinary team meeting of colorectal surgeons, radiologists and endoscopists.
Endoscopic procedures
All endoscopic procedures were performed (using a standardized technique and under general anaesthesia) by a specialist endoscopist in a radiology room. Patients had previously received a rectal enema preparation. A gastroscope (GIF-1TQ160, Olympus America Inc., Center Valley, PA, USA) was used for all endoscopic procedures. Under general anaesthesia and with CO2 insufflation, the gastroscope was introduced carefully in order to visualize the anastomosis. Next, the orifice of the fistula was dilated with a 0.035-in. guide wire (Tracer Metro ® Direct™, Wilson-Cook Medical, Bloomington, IN, USA), in order to drain the perianastomotic abscess as much as possible. A DPS (Zimmon® Biliary Stent, Cook Ireland Ltd, Limerick, Ireland) was inserted through the orifice of the fistula (between the peritoneal cavity and the lumen of the colon or rectum). It should be noted that the DPS never sticks out through the anus but always remains in the rectal lumen (given the fistula’s location). No surgical drains were placed with a transanal approach (either in the initial procedure or for fistula management). If a surgical or external radiological drain was present, it was removed during the procedure or in the following days, in order to allow reverse flow of the fluid into the lumen of the colon. The placement was checked radiologically at the end of procedure. A CT scan with an intra-luminal contrast enema was used to control the efficacy of the internal drainage six weeks later and could be repeated every six weeks until the AL had disappeared.
Definition of success
Six weeks after DPS insertion, each patient was hospitalized for a detailed clinical examination and laboratory tests for signs of inflammation. Each patient also underwent a contrast-enhanced CT scan for abscesses and fistulae. If none of these investigations evidenced signs of persistent sepsis, the DPS was removed (corresponding to success for treatment with a DPS). If there was persistent collection but no clinical signs of sepsis and the DPS appeared not be located within the abscess, another attempt to place DPS was made (Fig. 2).
Results
From September 2011 to December 2014, we recorded 24 consecutive patients with grade B AL. Nine were treated exclusively with a DPS (37.5 %), 10 were treated exclusively with radiological drainage (41.7 %), and five were treated first with radiological drainage and then with a DPS (20.8 %). Hence, the study population comprised 14 patients (58.3 %) (Tables 1, 2).
In the ten patients with radiological drainage, the median duration of drainage was 6 days (range 5–8); drainage was successful in all cases, and there was no recurrence of the abscess. In the four patients with a stoma, the median time to closure was 10 months (range 6–11).
For patients treated with a DPS (i.e. the study population), the mean age was 58 years (range 33–75) and the mean BMI was 21 ± 9.5 kg/m2 (range 23.4–47) (Table 1). Surgical indications, procedures and signs leading to the diagnosis of AL are summarized in Table 1.
Endpoints
Primary endpoint
Feasibility of DPS placement
DPS placement was feasible in 92.8 % of cases (n = 13 out of 14). The sites of the anastomoses drained with DPSs are summarized in Table 3. The median time interval between the diagnosis of AL and DPS placement was 13 days (range 7–171). In one patient, the fistula track was not detected during the endoscopic procedure, leading to the failure of endoscopic management. However, the patient’s abscess disappeared after a prolonged course of antibiotics.
The success of endoscopic management
The overall success rate for endoscopic management was 78.5 % (n = 11 out of 14) with a median number of endoscopic procedures per patient of 2 (range 2–4) and a median number of DPS per patient of 2 (range 2–3). The median length of hospitalization after DPS placement was 5 days (range 3–17). The mean duration of drainage through a DPS was 62 days (range 28–181). Adverse events were observed in two patients (migration of the DPS requiring its replacement, in both cases). In one patient, the presence of a large anastomotic hole meant that the initial placement of a DPS had to be complemented by the insertion of a self-expanding metal stent with an anti-reflux valve (HANAROSTENT®, Life Partners Europe, Bagnolet, France) (Fig. 2). The DPSs were well tolerated, and there was no need for drain removal due to discomfort or rectal syndrome.
Secondary endpoints
Management of associated radiological drains
Five patients (35.7 %) underwent radiological drainage and DPS placement. In four cases, the radiological drain was removed during the endoscopic procedure. In the fifth case, the external drain was only removed 5 days later because of the presence of pus in the drain at the time of the endoscopic procedure (endoscopist’ decision).
Management of the failure of DPS placement
Treatment of AL with a DPS failed in three patients. One patient underwent emergency surgery for pelviperitonitis (Hartmann procedure) 17 days after DPS placement. The aetiology of the pelviperitonitis remains unclear; the condition was not associated with massive pneumoperitoneum and did not appear to be related to endoscopic management of AL. The second patient underwent an abdominoperineal excision for chronic sepsis of the pelvis, and the third patient was successfully treated with antibiotics.
Long-term outcomes
Eight of the ten patients with stoma (80 %) achieved closure. Of the seven patients operated on for colorectal cancer, four required adjuvant chemotherapy. All four were able to undergo chemotherapy with the DPS in place. The DPS was removed at the end of the course of chemotherapy.
Discussion
Anastomotic leakage is one of the most frequent and difficult-to-manage complications of colorectal surgery. The International Anastomotic Leak Study Group has developed an algorithm for the management of AL with pelvic abscesses after colorectal surgery. For patients with grade B AL and a perianastomotic abscesses greater than 3 cm in size, radiologically guided drainage and intravenous broad-spectrum antibiotic therapy (effective against Gram-negative and anaerobic organisms) can be considered [14, 15]. In the event of failure, the treatment options include: (i) laparotomic reoperation for perianastomotic drainage (if the abscess is unresolved), (ii) end colostomy if there is a major defect and (iii) a diverting stoma with perianastomotic drainage if there is a minor defect. The role of endoscopic treatment remains unclear and mainly includes glue therapy at least 6 months after the initial surgery [1, 16–18]. Nevertheless, some series have described conservative treatment options (stent, glue or endoclip placement with endoscopic guidance) with much the same success rate as surgery [19–23].
In the present study, we found that early endoscopic management (at a median of 13 days after diagnosis) is feasible in 92.8 % of patients with grade B AL. The procedure was effective in 78.5 % of cases. The use of a DPS also enabled the early removal of external drains and is thus an option after the failure of radiological drainage. The use of a DPS also enabled early discharge (with a median length of stay of 5 days (range 3–17)) and a high stoma closure rate (80 %) 7 months after DPS placement, on average (range 5–10). The DPS can also be used as a first-line treatment, as a way of avoiding external drainage. Furthermore, we did not observe any complications related to endoscopic treatment; this might be due to the great experience of endoscopists who performed the DPS placement (with CO2 insufflation used to lower the tension in the anastomosis).
In a survey of practice sent to individual members of the Dutch Society of Gastrointestinal Surgery, 60 % of the respondees stated that they protect anastomosis by stoma (for grade B AL) and 40 % would resect the anastomosis [24]. This would lead to greater loss of anastomosis than the strategy proposed here, which argues in favour of the use of DPSs for the management of grade B AL.
AL is also associated with poor overall survival. The reason remains unclear but could be related to worse access to chemotherapy or to local inflammation [10]. In the present study, we found that patients who required chemotherapy were always able to receive it. This point is of value because patients in whom conservative treatment fails may undergo reoperation and thus cannot receive chemotherapy because of the post-operative alteration in their general status. This and other points of interest should be studied in larger series, in order to evaluate the impact on survival of access to chemotherapy in patients with AL.
Conclusion
The treatment of AL requires multidisciplinary collaboration to save the anastomosis. The placement of a DPS under endoscopic guidance is associated with a good AL healing rate and may be an alternative to repeat laparotomy when radiological drainage is unfeasible or inefficient.
Abbreviations
- AL:
-
Anastomotic leakage
- DPS:
-
Double-pigtail stent
References
Phitayakorn R, Delaney CP, Reynolds HL, Champagne BJ, Heriot AG, Neary P, Senagore AJ, International Anastomotic Leak Study Group (2008) Standardized algorithms for management anastomotic leaks and related abdominal and pelvic abscesses after colorectal surgery. World J Surg 32:1147–1156
Chambers WM, Mortensen NJ (2004) Postoperative leakage and abscess formation after colorectal surgery. Best Pract Res Clin Gastroenterol 18:865–880
Peel AL, Taylor EW (1991) Proposed definitions for the audit of postoperative infection: a discussion paper. Surgical Infection Study Group. Ann R Coll Surg Engl 73:385–388
Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, Holm T, Wong WD, Tiret E, Moriya Y, Laurberg S, den Dulk M, van de Velde C, Buchler MW (2010) Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery 147:339–351
Fielding LP, Stewart-Brown S, Blesovsky L, Kearney G (1980) Anastomotic integrity after operations for large-bowel cancer: a multicentre study. Br Med J 281:411–414
Ansari MZ, Collopy BT, Hart WG, Carson NJ, Chandraraj EJ (2000) In-hospital mortality and associated complications after bowel surgery in Victorian public hospitals. Aust N Z J Surg 70:6–10
Makela JT, Kiviniemi H, Laitnen S (2003) Risk factors for anastomotic leak after left-sided colorectal resection with rectal anastomosis. Dis Colon Rectum 46:653–660
van Koperen PJ, van der Zaag ES, Omloo JM, Slors JF, Bemelman WA (2011) The persisting presacral sinus after anastomotic leakage following anterior resection or restorative proctocolectomy. Colorectal Dis 13:26–29
den Dulk M, Smit M, Peeters KC, Kranenbarg EM, Rutten HJ, Wiggers T, Putter H, van de Velde CJ, Dutch Colorectal Cancer Group (2007) A multivariate analysis of limiting factors for stoma reversal in patients with rectal cancer entered into the total mesorectal excision (TME) trial: a retrospective study. Lancet Oncol 8:297–303
Marra F, Steffen T, Kalak N, Warschkow R, Tarantino I, Lange J, Zünd M (2009) Anastomotic leakage as a risk factor for the long-term outcome after curative resection of colon cancer. Eur J Surg Oncol 35:1060–1064
Chung IH, Kim HW, Lee DK (2011) Endoscopic removal of a migrated cystogastrostomy double pigtail stent through a pancreatico-duodenal fistula tract. J Interv Gastroenterol 1:142–144
Bartoli E, Rebibo L, Robert B, Fumery M, Delcenserie R, Regimbeau JM (2014) Efficacy of the double-pigtail stent as a conservative treatment for grade B pancreatic fistula after pancreatoduodenectomy with pancreatogastric anastomosis. Surg Endosc 28:1528–1534
Pequignot A, Fuks D, Verhaeghe P, Dhahri A, Brehant O, Bartoli E, Delcenserie R, Yzet T, Regimbeau JM (2012) Is there a place for pigtail drains in the management of gastric leaks after laparoscopic sleeve gastrectomy? Obes Surg 22:712–720
Robert B, Chivot C, Fuks D, Gondry-Jouet C, Regimbeau JM, Yzet T (2013) Percutaneous, computed tomography-guided drainage of deep pelvic abscesses via a transgluteal approach: a report on 30 cases and a review of the literature. Abdom Imaging 38:285–289
Robert B, Yzet T, Regimbeau JM (2013) Radiologic drainage of post-operative collections and abscesses. J Visc Surg 150:S11–S18
Truong S, Böhm G, Klinge U, Stumpf M, Schumpelick V (2004) Results after endoscopic treatment of postoperative upper gastrointestinal fistulas and leaks using combined Vicryl plug and fibrin glue. Surg Endosc 18:1105–1108
Bonanomi G, Prince JM, McSteen F, Schauer PR, Hamad GG (2004) Sealing effect of fibrin glue on the healing of gastrointestinal anastomoses: implications for the endoscopic treatment of leaks. Surg Endosc 18:1620–1624
Fuks D, Bréhant O, Dumont F, Viart L, Manaouil D, Bartoli E, Yzet T, Mauvais F, Regimbeau JM (2007) Tissue adhesive treatment of persistent recto-cutaneous fistula following Hartmann procedure. J Chir (Paris) 144:35–38
Chopra SS, Mrak K, Hünerbein M (2009) The effect of endoscopic treatment on healing of anastomotic leaks after anterior resection of rectal cancer. Surgery 145:182–188
Liu Z, Li C, Wang J, Liu Y (2012) Endoscopic incision of a rectal anastomotic fistula wall following pancolectomy with ileorectal anastomosis.Endoscopy 44 UCTN:E69-70
van Koperen PJ, van Berge Henegouwen MI, Rosman C, Bakker CM, Heres P, Slors JF, Bemelman WA (2009) The Dutch multicenter experience of the endo-sponge treatment for anastomotic leakage after colorectal surgery. Surg Endosc 23:1379–1383
Ibis M, Beyazit Y, Onal IK, Kurt M, Parlak E (2010) Successful endoscopic closure of anastomotic leakage following anterior resection of the rectum by endoclip application. Am J Gastroenterol 105:1447–1448
Pérez Roldán F, González Carro P, Villafáñez García MC, Aoufi Rabih S, Legaz Huidobro ML, Bernardos Martín E, Villanueva Hernández R, Tebar Romero E, Ruiz Carrillo F (2013) Endoscopic treatment of postsurgical colorectal anastomotic leak (with videos). Gastrointest Endosc 77:967–971
Daams F, Slieker JC, Tedja A, Karsten TM, Lange JF (2012) Treatment of colorectal anastomotic leakage: results of a questionnaire amongst members of the Dutch Society of Gastrointestinal Surgery. Dig Surg 29:516–521
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Christelle Blot, Charles Sabbagh, Lionel Rebibo, Franck Brazier, Cyril Chivot, Mathurin Fumery and Jean-Marc Regimbeau have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Blot, C., Sabbagh, C., Rebibo, L. et al. Use of transanastomotic double-pigtail stents in the management of grade B colorectal leakage: a pilot feasibility study. Surg Endosc 30, 1869–1875 (2016). https://doi.org/10.1007/s00464-015-4404-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4404-6