Abstract
Background
The benefits and feasibility of laparoscopic surgery for remnant gastric cancer are still unclear. The purpose of this study was to describe the detailed procedure and to evaluate the clinical short-term outcomes of laparoscopic total gastrectomy (LTG) compared with open total gastrectomy (OTG) for remnant gastric cancer (RGC).
Methods
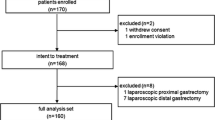
Of 1,247 consecutive patients who underwent gastrectomy for gastric cancer in our department at Kyushu University Hospital from January 1996 to May 2012, 22 patients who underwent successful curative resection of RGC with precise nodal dissection were enrolled in this study. Twelve patients underwent LTG and the remaining ten patients underwent OTG. We analyzed the clinical short-term outcomes of LTG and compared the results between LTG and OTG groups to evaluate the safety and feasibility of LTG.
Results
Twelve patients with RGC successfully underwent LTG without open conversion and morbidity. The mean operation time of LTG, 362.3 ± 68.4 min, was significantly longer than that of OTG (p = 0.0176), but the mean blood loss of LTG, 65.8 ± 62 g, was smaller than that of OTG (p < 0.01). The mean postoperative times to resumption of water and food intake were significantly shorter in the LTG group than in the OTG group (p < 0.01). The overall 3-year survival rate was comparable between the LTG and OTG groups (77.8 vs. 100 %; p = 0.9406).
Conclusions
This study shows that LTG is a feasible and reliable procedure for the treatment of RGC in terms of short-term outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastric cancer is the second most common cause of cancer death worldwide [1]. Surgical resection remains the only potentially curative treatment. Earlier detection due to advances in diagnostic modalities and the development of adjuvant therapy, such as oral S-1, have improved the prognosis of patients with gastric cancer. Distal gastrectomy is the most commonly performed surgical procedure, and there are an increasing number of cured patients with remnant stomach who are at risk of developing a second gastric cancer. Remnant gastric carcinoma (RGC) has been reported to occur in 2–3 % of patients who have undergone gastrectomy for carcinoma or benign disease and was reported to account for 1.8 % of all gastric cancers in a large series [2–5].
Surgical treatment is more difficult for RGC than for primary gastric cancer due to adhesions to adjacent organs, displacement of anatomical structures, and changes in lymphatic flow. There is an increased risk of tumor invasion into the hepatic parenchyma due to adhesions between the remnant stomach and the left lobe of the liver, requiring combined resection of the liver for cure. Displacement of anatomical structures makes it difficult to remove the remnant stomach and to dissect the connective tissues around the arteries, especially around the celiac artery. Lymph node dissection should be modified to account for changes in lymphatic flow and may need to be extended.
Laparoscopic gastrectomy has gained acceptance gradually for the treatment of primary gastric carcinoma because of the potential benefits in terms of being less invasive and having a shorter recovery time but has generally been considered contraindicated in patients who have undergone previous open upper abdominal surgery [6–8]. Recently, Tokunaga et al. [9]. reported that laparoscopy-assisted gastrectomy could successfully be performed by an experienced laparoscopic surgeon in patients with previous open upper abdominal surgery excluding gastrectomy. However, few reports describe laparoscopic completion total gastrectomy (LTG) after open or laparoscopic gastrectomy [10–14]. The benefits and feasibility of laparoscopic surgery for RGC are still unclear. The purpose of this study was to describe the detailed procedure and evaluate the safety and feasibility as well as clinical short-term outcomes of LTG for RGC.
Patients and methods
Of 1,247 consecutive patients recorded in a prospectively maintained gastric cancer database in our department at Kyushu University Hospital from January 1996 to May 2012, 27 underwent completion total gastrectomy for RGC. Twenty-two of these 27 patients underwent successful curative resection with precise nodal dissection and were enrolled in this study. The other five patients were diagnosed with stage IV carcinoma because of hepatic metastasis, peritoneal dissemination, or distant lymph nodal metastasis, and underwent palliative surgery for bleeding or obstruction.
We started performing laparoscopy-assisted distal gastrectomy for gastric cancer in 1996. We subsequently expanded our indications for laparoscopic surgery to include total gastrectomy in 2002 and completion total remnant gastrectomy in 2005. We started performing laparoscopic total gastrectomy for advanced gastric cancer in July 2007. Thereafter, LTG was indicated for all operable RGC.
LTG was performed in 1 patient in July 2005 and in 11 patients from December 2006 to May 2012. The remaining ten patients underwent OTG from June 1996 to February 2006.
Preoperative clinical assessments, including clinical classification of tumor depth (cT) and nodal involvement (cN), were performed by upper gastrointestinal radiography, esophagogastroduodenoscopy, endoscopic ultrasonography, abdominal ultrasonography, and computed tomography according to the TNM staging system.
Surgical procedures
Under general anesthesia, the patient was placed in the supine position with the legs slightly apart. The operator stood on the patient’s right side, the first assistant on the patient’s left side, and the camera operator stood between the patient’s legs. First, a 12-mm trocar was placed in the left lateral abdomen, to avoid injury to the intestines during CO2 insufflation of the abdominal cavity to a pressure of 10 mmHg. The intestines or greater omentum usually were severely adherent to the previous surgical incision scar or the right upper abdominal wall (Fig. 1A, B). After insufflation, a 5-mm trocar was placed in the left hypochondriac region and a 12-mm trocar was placed at the umbilicus or in the right lateral abdomen in an area without adhesions. These three trocars were used to perform adhesiolysis at the previous incision site and the right upper abdominal wall. Further trocars were placed until there were five trocars in total: 12-mm trocars in the left lateral abdomen, right lateral abdomen, and the region of the umbilicus; and 5-mm trocars in the left and right hypochondriac regions (Fig. 1C, D).
Patients who underwent Billroth I reconstruction during the initial surgery
After division of the adhesions between the abdominal wall and the intestines, the gastroduodenal anastomosis was exposed. The connective tissues between the transverse colon and the greater curvature of the stomach were divided. Because the inferior surface of the left lateral segment of the liver was usually severely adherent to the superior surface of the gastric remnant, this area was dissected carefully to avoid injury to the gastric wall (Fig. 2A). Adhesions between of the posterior wall of the stomach and the mesocolon and the pancreas were divided. After separating the gastric wall and duodenal wall from the pancreatic parenchyma, the gastroduodenal anastomosis was completely exposed, and tape was placed around the duodenum for traction (Fig. 2B). The duodenum was divided at a small distance from the anastomosis using a linear stapler (Fig. 2C). The gastric remnant was retracted upwards to obtain an adequate view for lymphadenectomy. The common hepatic artery, celiac trunk, and splenic artery were exposed, and the lymph nodes along these vessels were dissected. If necessary, the lymph nodes at the splenic hilum also were dissected. If the left gastric vessels had been left intact during the initial surgery, they were now divided. The esophagus was then exposed and was divided on the oral side of the esophagogastric junction, at a sufficient distance from the tumor. The resected specimen was removed through the umbilical incision, which was enlarged to approximately 3 cm. Roux-en-Y reconstruction was performed with an isoperistaltic 40-cm Roux limb divided at 30 cm from the duodenojejunal junction. The Roux limb was ascended through the antecolic route. Esophagojejunal anastomosis was performed using a linear stapler. Side-to-side jejunojejunostomy was performed at 40 cm from the esophagojejunostomy using a linear stapler. The jejunojejunostomy and Petersen’s mesenteric defect were closed with continuous sutures.
Patients who underwent Billroth II reconstruction during the initial surgery
Previous Billroth II (B-II) reconstruction was usually via the retrocolic route. The gastrojejunal anastomosis was clearly identified behind the mesocolon (Fig. 3A–C), and the afferent and efferent limbs were sequentially divided at an appropriate distance from the gastrojejunal anastomosis using a linear stapler (Fig. 3D). The stumps of the jejunum were pulled up to the front of the mesocolon.
The gastric remnant was retracted upwards for lymphadenectomy. The left gastric vessels had usually been left intact during the initial surgery and were now divided. The lymph nodes along the greater curvature were dissected and omentectomy was performed. The lymph nodes along common hepatic artery, celiac trunk, and splenic artery were dissected (Fig. 4A, B). After excision of the lesser omentum adjacent to the left lateral segment of the liver, the esophagus was exposed and divided on the oral side of the esophagogastric junction. In general, lymphadenectomy was performed according to the concept of D2 nodal dissection for primary gastric cancer. If there was tumor invasion into the jejunal wall, the mesenteric lymph nodes close to the anastomosis also were dissected. After removal of the resected specimen, Roux-en-Y reconstruction was performed as described above.
Statistical analysis
Perioperative clinical data were collected from the patient records. All values are expressed as mean ± standard deviation. Categorical variables were compared using the unpaired Chi-square test, and continuous variables were compared using the Student’s t test or Mann–Whitney U test. Patient survival was calculated using the Kaplan–Meier method, and the significance of differences between curves was analyzed using the log-rank test. A p value <0.05 was considered significant. Statistical analyses were performed using JMP version 8.0 (SAS Institute, Cary, NC, USA).
Results
Clinicopathological characteristics of the LTG group
The clinical and surgical characteristics of the 12 patients in the LTG group are shown in Table 1. The male to female ratio was 10:2 and the mean age of patients was 65.9 ± 6.8 years. Initial gastrectomy was for gastric cancer in five patients and peptic ulceration in seven. The initial surgery was laparoscopic in three patients and open in nine, including Billroth I (B-I) gastroduodenostomy in five patients, B-II gastrojejunostomy in six, and esophagogastrostomy in one. Four of five patients with B-I reconstruction had undergone their initial gastrectomy for cancer, and all six patients with B-II reconstruction had undergone their initial surgery for peptic ulceration. The mean time from the initial surgery to completion total gastrectomy was 26.7 ± 16.9 years. The mean operation time of the second operation in all 12 patients was 362.3 ± 68.4 min (365.8 ± 74.0 min in patients with B-I reconstruction and 363.8 ± 68.2 min in patients with B-II reconstruction; p = 0.9678). The mean estimated blood loss in all 12 patients was 68.5 ± 62 g (49.8 ± 27.8 g in patients with B-I reconstruction and 88.5 ± 76.2 g in patients with B-II reconstruction; p = 0.3549). There were no conversions to open surgery. The mean tumor diameter was 3.1 ± 2.1 cm. The tumor was located at the anastomotic site in six patients (50 %), at a nonanastomotic site in four (33.3 %), and at the gastric stump line in two (16.7 %). Nine resected RGCs were the superficial depressed type and three were the superficial elevated type. The mean number of lymph nodes harvested was 23.7 ± 10.7 (14.3 ± 7.1 in patients with B-I reconstruction and 28.5 ± 8.9 in patients with B-II reconstruction; p = 0.0433). In this study, reconstruction was highly related to the initial diagnosis; cancer or peptic ulcer, as described above. Therefore, the difference of the number of the retrieved lymph nodes between B-I and B-II patients depended on the initial diagnosis. Histological examination showed undifferentiated or signet-ring cell carcinoma in eight patients and differentiated or intestinal-type adenocarcinoma in four. Tumor stage was T1a in five patients (41.7 %), T1b in five (41.7 %), and T3 in two (16.7 %). The final stage was IA in ten patients (83.3 %) and IIA in two (16.7 %). The mean times to postoperative liquid and food intake were 2.5 ± 1.0 and 4.2 ± 0.8 days, respectively. The mean length of postoperative hospital stay was 11.3 ± 2.8 days (12.0 ± 3.6 days in patients with B-I reconstruction and 10.7 ± 2.1 days in patients with B-II reconstruction; p = 0.5069). There were no perioperative complications or deaths. The mean follow-up time was 39.1 (range 7.4–67.4) months. Ten of the 12 patients were still alive without relapse at the time of writing. One patient died of malignant lymphoma, and one died of metastatic disease in the brain and multiple lymph nodes around the aorta. Comparing surgical outcome of the patients in LTG group with regard to the type of reconstruction in the initial surgery, there were no significant differences between B-I and B-II group.
Comparisons between the LTG and OTG groups
Comparisons of patient characteristics between the LTG and OTG groups are shown in Tables 2, 3, and 4. There were no significant differences in age or sex distribution between the two groups. The mean body mass index was 20.7 ± 2.9 kg/m2 in the LTG group, which was not significantly different from the OTG group. The mean time from the initial surgery to completion total gastrectomy was 14.9 ± 12.9 years in patients with previous malignant gastric disease and 38.7 ± 8.3 years in patients with previous benign disease (p = 0.00007). The mean time from the initial surgery to completion total gastrectomy was comparable between the LTG and OTG groups (26.7 ± 16.9 vs. 21.8 ± 16.3 years; p = 0.9084). The proportions of the type of initial gastrectomy, type of reconstruction, and initial gastric disease were almost the same in the LTG and OTG groups. Seven of the 12 patients (58.3 %) in the LTG group had medically treated comorbidities at the time of the second operation, including diabetes mellitus, hypertension, arrhythmia, and interstitial pneumonia; and six of the ten patients (60 %) in the OTG group had comorbidities, including liver cirrhosis, diabetes mellitus, hypertension, arrhythmia, and dilated cardiomyopathy. There was no significant difference in the comorbidity rate between the two groups (p = 0.7216). Malignant neoplasms other than gastric cancer were diagnosed in 1 of the 12 patients (8.3 %) in the LTG group and three of the ten patients (30 %) in the OTG group (p = 0.3133). Operation time was significantly longer in the LTG group than in the OTG group (362.3 ± 68.4 vs. 270.5 ± 94.9 min; p = 0.0176). The mean estimated intraoperative blood loss was 68.5 ± 62 g in the LTG group and 746.3 ± 577.1 g in the OTG group (p = 0.0006). The mean number of lymph nodes harvested was 23.7 ± 10.7 in LTG group, which was comparable to that in OTG group (15.7 ± 7.6, p = 0.1301). Postoperative hemorrhage occurred in two of the ten patients (20 %) in the OTG group. There were no intra-abdominal complications in the LTG group. There were no cases of anastomotic leakage, postoperative intestinal stasis, pancreatic leakage, or perioperative death in either group. The mean time to postoperative resumption of water intake was significantly shorter in the LTG group than in the OTG group (2.5 ± 1.0 vs. 6.9 ± 2.4 days; p = 0.00002), as was the mean time to postoperative resumption of food intake (4.2 ± 0.8 vs. 8.7 ± 1.6 days; p < 0.0001). The length of postoperative hospital stay was significantly shorter in the LTG group than in the OTG group (11.3 ± 2.8 vs. 24.9 ± 10 days; p = 0.0023).
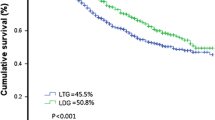
The mean follow-up time was 39.1 ± 20.5 months in the LTG group and 62.7 ± 39.8 months in the OTG group (p = 0.0913). In the LTG group, ten patients had TMN stage IA and two had TMN stage IIA. In the OTG group, five patients had TMN stage IA, one had TMN stage IIA, two had TMN stage IIA, and two had TMN stage IIIA. There was no significant difference in the distribution of TMN stages between the LTG and OTG groups. One of the ten patients in the OTG group died of recurrent disease at 51.2 months after the second operation, and three died of other disease (two of liver cirrhosis and one of right lung cancer). The overall 3-year survival rate was 77.8 % in the LTG group and 100 % in the OTG group. The overall 5-year survival rate was 72.9 % in the OTG group. These survival rates were comparable between the two groups (p = 0.9406; Fig. 5).
Discussion
Laparoscopic surgery is an established standard surgical treatment option for early gastric cancer, especially in Japan and Korea [6–8]. For primary gastric cancer, laparoscopic gastrectomy has been reported to have some advantages compared with open gastrectomy, such as a smaller wound, smaller amount of blood loss during surgery, less pain, and shorter postoperative hospital stay. It is possible that laparoscopic surgery for RGC also may have advantages compared with open surgery, but no studies of the feasibility and safety of LTG compared with OTG have been reported.
RGC occurs in 2–3 % of gastric remnants [2–4] and has been reported to account for 1–2 % of all gastric cancers [5, 15]. RGC accounted for 2.1 % of gastric cancers treated in our department. Although this is only a small proportion of our gastric cancer patients, treatment of RGC is important and presents some specific problems. The mainstay of treatment for RGC is surgical resection, as for primary gastric cancer [16]. Surgical treatment is more difficult in patients with RGC than in patients with primary gastric cancer, and LTG is more technically demanding than OTG. In the present study, almost patients (80 %) with B-I reconstruction had undergone their initial gastrectomy for cancer and lymphadenectomy of the supra- and infra-pyloric and suprapancreatic nodes, resulting in severe adhesions in those areas.
Our series highlights some important technical points: (1) because of severe adhesions between the pancreatic parenchyma and the posterior wall of the duodenum, adhesiolysis in this area should be performed carefully to avoid injury to the pancreatic parenchyma and duodenal wall; (2) suprapancreatic dissection should be performed carefully to avoid injury to the common hepatic artery, splenic artery, and especially the left gastric artery if it was left intact during the initial surgery. After isolation of the common hepatic artery near the origin of the gastroduodenal artery, dissection should be continued along the common hepatic artery to the left gastric artery; and (3) because there are usually severe adhesions between the dorsal surface of the left lateral segment of the liver and the anterior surface of the remnant gastric wall, dissection should be performed carefully in this area to avoid injury to the gastric wall. Although adhesion of the gastric wall to the hepatic and pancreatic parenchyma is less severe in patients with B-II reconstruction than in those with B-I reconstruction, the jejunal and gastric walls are severely adherent to the mesocolon in patients with B-II reconstruction, and it is important to avoid injury to the colonic arteries during adhesiolysis. We followed these precautions and successfully performed LTG without perioperative massive bleeding, pancreatic leakage, anastomotic leakage, or conversion to open surgery.
Because of the technical difficulties and the small number of patients treated, few reports describe laparoscopy-assisted completion total gastrectomy and LTG [10–14]. Yamada et al. [10]. first reported the technical feasibility of laparoscopy-assisted completion total gastrectomy for early cancer in 2005. We started to perform LTG for RGC in 2005 and successfully performed this procedure in 12 patients from 2005 to 2012. We analyzed the short-term results of LTG compared with OTG. Although the mean operation time was significantly longer in the LTG group, the mean blood loss was significantly smaller than in the OTG group. The mean postoperative times to resumption of water and food intake were significantly shorter in the LTG group than in the OTG group. The length of postoperative hospital stay also was significantly shorter in the LTG group than in the OTG group. The morbidity rate was not increased by laparoscopic surgery. The results of our study indicate that LTG, regardless of whether the type of reconstruction is B-I or B-II in the initial operation, is feasible and is less invasive than OTG, with favorable short-term outcomes.
One of the 12 patients who underwent LTG was diagnosed with metastatic disease in the brain and the mediastinal and para-aortic lymph nodes at 6 months after surgery and died of disease at 31 months after surgery. The prognosis of advanced RGC has been reported to be worse than that of advanced primary gastric cancer [15]. This may be because the disruption of lymphatic channels during surgery for primary gastric cancer causes substantial changes to lymphatic flow from the gastric remnant, making surgical control of RGC with nodal disease difficult. In our patient, extensive metastasis to the lymph nodes and brain might have occurred at an early stage because of the substantial changes to lymphatic and blood flow after the initial surgery. Adjuvant chemotherapy therefore may be important for the treatment of advanced RGC.
We acknowledge some limitations of this retrospective study. The number of patients included was relatively small, even though the incidence of remnant gastric cancer was very low, and there may have been selection bias between the LTG and OTG groups. However, we believe that the present study demonstrate useful clinical aspects for the treatment of RGC, because the incident of remnant gastric cancer will increase along with early detection of gastric cancer and with its’ good long-term survival.
In conclusion, we successfully performed LTG in 12 patients with RGC without open conversion and morbidity. LTG is considered to be technically acceptable and feasible for the treatment of RGC, with favorable short-term outcomes.
References
Ferlay J, Shin HR, Bray F et al (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–2917
Ovaska JT, Havia TV, Kujari HP (1986) Risk of gastric stump carcinoma after gastric resection for benign ulcer disease. Ann Chir Gynaecol 75:192–195
Welvaart K, Warnsinck HM (1982) The incidence of carcinoma of the gastric remnant. J Surg Oncol 21:104–106
Nozaki I, Nasu J, Kubo Y et al (2010) Risk factors for metachronous gastric cancer in the remnant stomach after early cancer surgery. World J Surg 34:1548–1554
Kaneko K, Kondo H, Saito D et al (1998) Early gastric stump cancer following distal gastrectomy. Gut 43:342–344
Adachi Y, Shiraishi N, Shiromizu A et al (2000) Laparoscopy-assisted Billroth I gastrectomy compared with conventional open gastrectomy. Arch Surg 135:806–810
Shimizu S, Uchiyama A, Mizumoto K et al (2000) Laparoscopically assisted distal gastrectomy for early gastric cancer: Is it superior to open surgery? Surg Endosc 14:27–31
Lee SI, Choi YS, Park DJ et al (2006) Comparative study of laparoscopy-assisted distal gastrectomy and open distal gastrectomy. J Am Coll Surg 202:874–880
Tokunaga M, Hiki N, Fukunaga T et al (2010) Laparoscopy-assisted gastrectomy for patients with earlier upper abdominal open surgery. Surg Laparosc Endosc Percutan Tech 20:16–19
Yamada H, Kojima K, Yamashita T et al (2005) Laparoscopy-assisted resection of gastric remnant cancer. Surg Laparosc Endosc Percutan Tech 15:226–229
Song J, Kim JY, Kim S et al (2009) Laparoscopic completion total gastrectomy in remnant gastric cancer: technical detail and experience of two cases. Hepatogastroenterology 56:1249–1252
Corcione F, Pirozzi F, Marzano E et al (2008) Laparoscopic approach to gastric remnant-stump: our initial successful experience on 3 cases. Surg Laparosc Endosc Percutan Tech 18:502–505
Qian F, Yu PW, Hao YX et al (2010) Laparoscopy-assisted resection for gastric stump cancer and gastric stump recurrent cancer: a report of 15 cases. Surg Endosc 24:3205–3209
Shinohara T, Hanyu N, Tanaka Y et al (2012) Totally laparoscopic complete resection of the remnant stomach for gastric cancer. Langenbecks Arch Surg 398:341–345
Ohashi M, Katai H, Fukagawa T et al (2007) Cancer of the gastric stump following distal gastrectomy for cancer. Br J Surg 94:92–95
Sasako M, Maruyama K, Kinoshita T et al (1991) Surgical treatment of carcinoma of the gastric stump. Br J Surg 78:822–824
Disclosures
Eishi Nagai, Kohei Nakata, Kenoki Ohuchida, Yoshihiro Miyasaka, Shuji Shimizu and Masao Tanaka have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagai, E., Nakata, K., Ohuchida, K. et al. Laparoscopic total gastrectomy for remnant gastric cancer: feasibility study. Surg Endosc 28, 289–296 (2014). https://doi.org/10.1007/s00464-013-3186-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-013-3186-y