Abstract
Background
Hyperinsulinemic hypoglycemia is common after Roux-en-Y gastric bypass (RYGB) and may result in weight regain. The purpose of our investigation was to compare the effect of RYGB, vertical sleeve gastrectomy (VSG), and duodenal switch (DS) on insulin and glucose response to carbohydrate challenge.
Methods
Patients meeting National Institutes of Health criteria for bariatric surgery selected their bariatric procedure after evaluation and education in this prospective nonrandomized study. Preoperatively and at 6, 9, and 12 months’ follow-up, patients underwent blood draw to determine levels of fasting glucose, fasting insulin, glycated hemoglobin (HbA1c), C-peptide, and 2-h oral glucose challenge test. Homoeostatic Model Assessment (HOMA)-IR, fasting to 1-h and 1- to 2-h ratios of glucose and insulin, were calculated. Statistical analysis was performed using ANOVA and Student’s paired t test. All procedures were performed via a laparoscopic technique at a single institution.
Results
Data from a total of 38 patients (13 RYGB, 12 VSG, 13 DS) were available for analysis. At baseline, all groups were similar; the only statistically significant difference was that DS patients had a higher preoperative weight and body mass index (BMI). All operations caused weight loss (BMI 47.7 ± 10–30.7 ± 6.4 kg/m2 in RYGB; 45.7 ± 8.5–31.1 ± 5.5 kg/m2 in VSG; 55.9 ± 11.4–27.5 ± 5.6 kg/m2 in DS), reduction of fasting glucose, and improved insulin sensitivity. RYGB patients had a rapid rise in glucose with an accompanying rise in 1-h insulin to a level that exceeded preoperative levels. This was followed by a rapid decrease in glucose level. In comparison, DS patients had a lower increase in glucose and 1-h insulin, and the lowest HbA1c. These differences were statistically significant at various data points. For VSG, the results were intermediary.
Conclusions
Compared to gastric bypass, DS results in greater weight loss and improves insulin sensitivity and glucose homeostasis without causing a hyperinsulinemic response. Because the response to challenge after VSG is intermediary, pyloric preservation alone cannot account for this difference.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Bariatric surgery is considered the most effective treatment for severe and morbid obesity [1]. Roux-en-Y gastric bypass (RYGB) has been the most popular stapling procedure in bariatric surgery, and many suggest that it is the gold standard. However, an increasing number of reports have shown that gastric bypass results in hyperinsulinemic hypoglycemia [2–4]. In fact, entities that were rarely described, such as non-insulinoma-pancreatogenous syndrome [5] and nesidioblastosis [6], have been the subject of an increasing number of publications describing patients after gastric bypass.
There are many possible explanations for rising obesity, but a significant cause is an increase in simple carbohydrate consumption. It estimated that domestic consumption of simple carbohydrates has increased over 400 % [7]. Simple carbohydrates cause a rapid rise in blood glucose. This results in an insulin surge. Insulin is anabolic hormone and drives nutrients into cells. Preventing this response has become the cornerstone of medical weight loss and nutritional guidance [8]. To offset hunger, nutritionists suggest eating foods that are low on the glycemic index that produce a smaller rise in insulin production. This is the basis of Mediterranean-type diets and other low-carbohydrate plans.
This discrepancy appears to be counterintuitive. Medical weight loss emphases reduced insulin fluctuations, but the most common surgical procedure promotes fluctuation. The impact of oral glucose tolerance testing on RYGB has been previously tested by our group [4]. We demonstrated that abnormal glucose tolerance was extremely common and that more than 80 % of patients tested had reactive hypoglycemia. Many patients had both hyperglycemia and hypoglycemia. These findings have been confirmed by other investigators, and it has been clearly shown that even asymptomatic patients can have abnormal oral glucose challenge test (OGCT) results after gastric bypass [9].
In comparison, there are few data on OGCT after vertical sleeve gastrectomy (VSG) and duodenal switch (DS). Both procedures have been effective in treating weight loss and diabetes [10, 11]. DS offers the greatest weight loss—albeit, at least historically, with the highest chance of micronutrient or protein malnutrition [12]. It would seem logical that if a more physiologic response is elicited after glucose challenge, it may be beneficial for long-term weight management.
For these reasons, we decided to compare the three major stapling bariatric procedures when challenged by a liquid OGCT or solid high-carbohydrate mixed meal in a cohort of bariatric surgical patients.
Methods
Forty-five patients were enrolled into this nonrandomized prospective trial before undergoing bariatric surgery. Patients were 18 years of age or older and met the National Institutes of Health guidelines for bariatric surgery. All patients went through the standard seminar and educational program available as part of the Center of Excellence at Lenox Hill Hospital. Study enrollment was discussed after procedure selection was completed by the patients.
All patients enrolled into the study underwent baseline blood tests that included glycated hemoglobin (HbA1c), C-peptide level, and a 2-h OGCT using a liquid challenge of 100 g glucose with measurement of insulin levels.
All surgical procedures were performed at Lenox Hill Hospital by three surgeons. All were completed laparoscopically. Our techniques for each procedure have been well described and are consistent with accepted standard approaches. The RYGB is performed with an isolated gastric pouch of ~20 mL based on the lesser curvature, ending at the angle of His, with a 150-cm Roux limb and a 100-cm biliopancreatic limb. The VSG is performed over a 34F bougie starting 3–4 cm from the pylorus. The DS in our program is different from traditional reports in that we use a longer common channel at 125–150 cm with a 150-cm alimentary limb. The sleeve component of the DS is made using a 38F bougie.
All patients were followed and monitored according to our standard guidelines for their respective operations.
At 6 and 12 months after surgery, all patients had a 2-h OGCT with measurement of insulin and C-peptide levels along with glucose. At 9 months after surgery, the OGCT was performed using a solid mixed-meal muffin with the same 100-g glucose load as in the liquid form.
Using determined values, Homoeostatic Model Assessment (HOMA)-IR was calculated using a standardized equation. A modified quality-of-life questionnaire assessing common gastrointestinal symptoms was also administered at each study interval. All procedures and laboratory measurements were performed at Lenox Hill Hospital using standard techniques.
The ratios of baseline to 1- and 1- to 2-h glucose and insulin levels were calculated, as was the area under the curve (AUC) for glucose and insulin values.
The study design and informed consent were approved by the Lenox Hill institutional review board and registered at ClinicalTrials.gov.
Statistical analysis was performed by fixed-model ANOVA to compare results between RYGB, VSG, and DS. When ANOVA showed a statistical difference, Student’s t test was used to determine the difference between the subgroups.
Results
Forty-five patients were enrolled onto this study after the procedure of choice was selected. Seven patients were lost to follow-up. Data from 38 patients were used for analysis. A total of 13, 12, and 13 patients underwent RYGB, VSG, and DS, respectively. The three groups were demographically similar except that DS patients had a higher body mass index (BMI) (p < 0.05). There was no significant difference at baseline between fasting glucose, fasting insulin, or HbA1c.
All the operations successfully induced weight loss. At 1 year, the BMI fell from 47.7 ± 10 to 30.7 ± 6.4 kg/m2 in the RYGB group, 45.7 ± 8.5–31.1 ± 5.5 kg/m2 in the VSG group, and 55.9 ± 11.4–27.5 ± 5.6 kg/m2 in the DS group. This corresponds to 32.8 ± 7.2, 32.7 ± 7.9, and 49.8 ± 2.0 % of body weight loss, respectively, with a significantly greater loss in the DS group compared to the RYGB and VSG groups (p < 0.001). Similarly, there was a statistically significant decrease in fasting glucose and insulin for each surgical procedure (p < 0.001).
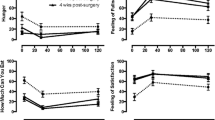
Glucose tolerance testing after surgery resulted in a consistent pattern for all three procedures (Table 1; Figs. 1, 2). RYGB resulted in rapid rise in glucose, and the 1-h insulin level was higher than at baseline at both 6 months and 1 year. With a solid muffin, the rise was lower but still more pronounced than VSG or DS. In comparison, DS had a much lower rise in glucose and 1-h insulin. The difference was statistically significant for 1-h insulin compared to RYGB at 6 months and the aggregate for all data points.
The response for VSG was intermediary to the response seen with DS and RYGB. The rise in insulin was less dramatic than RYGB but greater than DS.
A ratio of 1-h to preoperative glucose value showed the slope or degree of rise of glucose; this was calculated for each operation. Before surgery, there were no statistically significant differences in the 1-h to fasting glucose ratio (1.5 ± 0.5 for RYGB, 1.5 ± 0.3 for VSG and 1.5 ± 0.5 for DS; p = 0.97). At 6 months, however, this ratio increased to 1.8 ± 0.8, 1.6 ± 0.5, and 1.3 ± 0.3, respectively, with a statistically significant difference between RYGB and DS (p < 0.05). This trend remained the same at 9 months’ and 1 year’s follow-up (Table 2; Fig. 3).
Similarly, a ratio can be calculated that shows the rise in insulin after glucose ingestion. RYGB had the lowest 1-h to fasting insulin ratio at baseline. Postoperatively, after a rapid rise in glucose level, the insulin level increased by 23-, 20-, and 16-fold at 6, 9, and 12 months for RYGB; 11-, 7-, and 13-fold for VSG; and 11-, 6-, and 10-fold for DS. The difference was substantial and was statistically significant at 6 months (Table 2; Fig. 4).
As expected, the 1- to 2-h glucose ratio, which represents the response to spike of the insulin and the resulting decline in glucose, was the highest in RYGB group. This difference was significant when compared to DS at 6 months (p < 0.05) (Fig. 5), as was the aggregate of all postoperative values (p < 0.02).
All patients had improvement in hemoglobin HbA1c and insulin sensitivity (p < 0.001).
The AUC was calculated for all procedures (Table 3). All three procedures resulted in a significantly reduced glucose value under the curve (p < 0.05). However, this reduction was achieved for RYGB with enhanced insulin production, and insulin value under the curve increased.
Remarkably, DS had the lowest glucose and insulin AUC. The difference in insulin AUC was significant at 6 months (Figs. 1, 2).
A self-reported questionnaire of symptoms was also administered to patients at every glucose challenge. As an overall tendency, VSG patients reported the lowest level of weakness, dizziness, and nausea. These symptoms were highest in the RYGB group, not the DS group. Because this study was not powered to detect symptomatic differences, these results are not significant. However, they do show that at least in this cohort, DS patients do not experience symptoms of muscle weakness. These results are shown in Table 3.
Discussion
This study demonstrates that all three stapling procedures provide effective weight loss, reduce fasting insulin, improve glucose tolerance, reduce HbA1c, and lower fasting insulin levels. After glucose challenge, RYGB was more likely to result in a larger rise in blood glucose, a larger rise in 1-h insulin, and thus a greater reduction in blood glucose compared to DS. Results from VSG were intermediary. Although the sample size was small, the data were consistent at all time points, regardless of liquid or solid challenge.
Many consider RYGB the gold standard of bariatric procedures. RYGB involves creating a small pouch based on the lesser curvature of the stomach. The intestine is attached directly to the small pouch, and then a distal attachment created to restore bowel continuity. When glucose is given, it travels from the small pouch directly to the small bowel, bypassing the pyloric valve, duodenum, and proximal jejunum. As our study shows, RYGB causes a rapid rise in blood glucose, and insulin production is enhanced. It is believed that the increased insulin production is primarily caused by increased incretins [glucagon-like peptide-1 (GLP-1)], which are stimulated by food entering the small bowel directly [13]. McLaughlin et al. [14] showed that when a gastrostomy tube is placed into the remnant of a post-RYGB patient with abnormal glucose tolerance, glucose is normalized with liquid mixed meal into the remnant. Thus, the cause of the abnormal glucose challenge test is the result of nutrient delivery directly into the small bowel. Improved or enhanced insulin production is considered to be an important factor for improvement of glucose tolerance after RYGB. For those with insulin resistance, this response is potentially beneficial. For others, it may be detrimental. An increasing number of patients have required revision of their RYGB or even pancreatectomy for complications related to hypoglycemia [6].
Perhaps even more essential is the potential impact on long-term hunger and weight control. For years, it has been suggested that RYGB is the preferential procedure for those with a preference for simple carbohydrates and sweets. It was postulated that consumption of these foods would result in symptoms of dumping and thus create an aversion for such foods. However, no study has shown a positive correlation between dumping symptoms and weight loss. In fact, the two studies undertaken to determine the relationship between dumping and weight loss failed to demonstrate a difference [12, 15]. Despite educational efforts that attempt to get postbariatric surgical patients to avoid simple carbohydrates, it is highly unlikely that this is universally possible. As a result, a more likely consequence is a rapid rise in sugar, leading to enhanced insulin, followed by a fall in blood glucose and greater hunger. This cycle promoting intermeal hunger is what we believe to be a major contributor to recidivism after RYGB.
In comparison, the impact of DS on glucose tolerance appears different. To perform DS, the fundus and the greater curvature is resected and the duodenum is divided approximately 3 cm distal to the pyloric valve. A Roux-en-Y is created beneath the valve. In comparison to RYGB, the amount of bowel exposed to food, as well as the amount of bowel where bile and pancreatic juices mix with food, is reduced. Approximately 40–50 % of the total bowel is bypassed. Our study demonstrated that when DS patients are stimulated by glucose challenge, as compared to gastric bypass, there is a much smaller rise in blood glucose and 1-h insulin. This was found during all testing intervals and was consistent with liquid and solid challenge. Even more striking is the AUC data. All operations reduce AUC for glucose. DS causes the sharpest reduction. Most thought-provoking is that this reduction in glucose does not correlate to a higher insulin AUC. This suggests that the improvement is not the result of enhanced insulin production, as in RYGB, but perhaps a sharp reduction of insulin resistance peripherally. This finding may explain the greater efficacy seen for DS in diabetes [12]. Furthermore, Frenken et al. [16] have shown diabetes resolution after DS in a cohort of diabetics on high-dose insulin therapy for a lengthy period of time. It is in this group that RYGB has been shown to have the lowest efficacy [17].
Data from VSG seem to be intermediary between gastric bypass and DS. Our original hypothesis was that preservation of the pyloric valve would be an important component in regulating the impact of glucose challenge. Although it appears that there is a contribution from pyloric preservation, it does not appear to be the whole story. Whereas statistically significant differences can be seen between DS and RYGB, there was a tendency to difference, but not a statistically significant one, between DS and VSG or between VSG and RYGB. This indicates that the pylorus plays a role, but other factors must also contribute. Additionally, a large focus of conjecture for the role of bariatric surgery and the resolution of diabetes has been bypassing the duodenum (foregut theory) or stimulation of the distal intestine (hind gut theory) [18, 19]. Because both RYGB and DS bypass the duodenum and reduce transit time to the distal intestine, similar responses could be expected. Yet our study demonstrates differences. As a result, other factors are responsible for these findings. Possibilities include the rate of nutrient entry, a change in the microbiology of the gut, the impact of altered fat absorption, and a reduction of inflammatory factors that promote insulin resistance.
Although our study is to our knowledge the first to compare these operations, our findings have been corroborated. At all data points, and with both liquid and solid challenge, findings were similar. Additionally, other studies have shown similar results. Halperin et al. [9] studied gastric bypass patients with continuous glucose monitoring. With mixed meal challenge, their response was nearly identical to what was seen in our study. In contrast, Johansson et al. [20] studied the glucose and insulin response after a mixed meal in DS and normal controls. They found that in DS patients, the postprandial responses of glucose and insulin were virtually normal and there were no difference compared to controls.
The risk of vitamin deficiency, micronutrient deficiency, and diarrhea are the reasons given for not offering DS. The issues associated with DS can be mitigated by an alimentary limb and common channel of adequate length. In this study, a common channel of 125–150 cm and an alimentary limb of 150 cm were used, as is our practice standard. The important point is that the concerns raised about DS are directly related to the degree of intestinal bypass. Our study suggests that there may be profound advantages to a postpyloric bypass. Further investigation is required to define the ideal bowel lengths that can potentially maximize the anatomical advantages and minimize the risk of malabsorption. Additionally, Dorman et al. [12] have shown in a 5-year matched case–control trial that although resolution of comorbidities is greater with DS, long-term complications are not increased.
In conclusion, our study shows that in comparison to RYGB, DS regulates glucose without causing hyperinsulinemia. Surprisingly, the response to challenge of VSG patients is intermediary. This means that preservation of the pyloric valve is only partially responsible for these findings. Although our results are provocative, it is important to determine whether they will result in important clinical differences and change long-term outcomes. This can only be determined by further study on a larger number of patients. It is thus essential that randomized multisite trials be conducted to compare RYGB and DS on both diabetic and nondiabetic patients. Finally, caution should be used before calling or defining RYGB as a gold standard for bariatric procedures.
References
Sjostrom L (2013) Review of the key results from the Swedish Obese Subjects (SOS) trial—a prospective controlled intervention study of bariatric surgery. J Intern Med 273(3):219–234
Cui Y, Elahi D, Andersen DK (2011) Advances in the etiology and management of hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass. J Gastrointest Surg 15(10):1879–1888
Rabiee A, Magruder JT, Salas-Carrillo R, Carlson O, Egan JM, Askin FB, Elahi D, Andersen DK (2011) Hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass: unraveling the role of gut hormonal and pancreatic endocrine dysfunction. J Surg Res 167(2):199–205
Roslin MS, Oren JH, Polan BN, Damani T, Brauner R, Shah PC (2013) Abnormal glucose tolerance testing after gastric bypass. Surg Obes Relat Dis 9(1):26–31
Mathavan VK, Arregui M, Davis C, Singh K, Patel A, Meacham J (2010) Management of postgastric bypass noninsulinoma pancreatogenous hypoglycemia. Surg Endosc 24(10):2547–2555
Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV (2005) Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med 353(3):249–254
Lustig RH (2013) Fructose: it’s “alcohol without the buzz”. Adv Nutr 4(2):226–235
Ajala O, English P, Pinkney J (2013) Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr 97(3):505–516
Halperin F, Patti ME, Skow M, Bajwa M, Goldfine AB (2011) Continuous glucose monitoring for evaluation of glycemic excursions after gastric bypass. J Obes 2011:869536
Hedberg J, Sundbom M (2013) Superior weight loss and lower HbA1c 3 years after duodenal switch compared with Roux-en-Y gastric bypass—a randomized controlled trial. Surg Obes Relat Dis 8(3):338–343
Rawlins L, Rawlins MP, Brown CC, Schumacher DL (2013) Sleeve gastrectomy: 5-year outcomes of a single institution. Surg Obes Relat Dis 9(1):21–25
Dorman RB, Rasmus NF, al-Haddad BJ, Serrot FJ, Slusarek BM, Sampson BK, Buchwald H, Leslie DB, Ikramuddin S (2012) Benefits and complications of the duodenal switch/biliopancreatic diversion compared to the Roux-en-Y gastric bypass. Surgery 152(4):758–765
Salinari S, Bertuzzi A, Guidone C, Previti E, Rubino F, Mingrone G (2013) Insulin sensitivity and secretion changes after gastric bypass in normotolerant and diabetic obese subjects. Ann Surg 257(3):462–468
McLaughlin T, Peck M, Holst J, Deacon C (2010) Reversible hyperinsulinemic hypoglycemia after gastric bypass: a consequence of altered nutrient delivery. J Clin Endocrinol Metab 95(4):1851–1855
Mallory GN, Macgregor AM, Rand CS (1996) The influence of dumping on weight loss after gastric restrictive surgery for morbid obesity. Obes Surg 6(6):474–478
Frenken M, Cho EY, Karcz WK, Grueneberger J, Kuesters S (2011) Improvement of type 2 diabetes mellitus in obese and non-obese patients after the duodenal switch operation. J Obes 2011:860169
Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL (2012) Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 366(17):1567–1576
Mingrone G, Castagneto-Gissey L (2009) Mechanisms of early improvement/resolution of type 2 diabetes after bariatric surgery. Diabetes Metab 35(6 Pt 2):518–523
Yu H, Zheng X, Zhang Z (2013) Mechanism of Roux-en-Y gastric bypass treatment for type 2 diabetes in rats. J Gastrointest Surg 17(6):1073–1083
Johansson HE, Haenni A, Karlsson FA, Edén-Engström B, Ohrvall M, Sundbom M, Zethelius B (2010) Bileopancreatic diversion with duodenal switch lowers both early and late phases of glucose, insulin and proinsulin responses after meal. Obes Surg 20(5):549–558
Disclosures
This study was supported by a research Grant from Covidien. Dr. Roslin is an educational consultant for Johnson & Johnson and Covidien. He is also on the scientific advisory board for Surgiquest and Valentx. Dr. Shah is on the scientific advisory board of Stryker and Transenterix and is a consultant for Johnson & Johnson, Olympus, and Ethicon Endo-surgery. Drs. Brownlee and Dudiy and Ms. Weiskopf have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roslin, M.S., Dudiy, Y., Brownlee, A. et al. Response to glucose tolerance testing and solid high carbohydrate challenge: comparison between Roux-en-Y gastric bypass, vertical sleeve gastrectomy, and duodenal switch. Surg Endosc 28, 91–99 (2014). https://doi.org/10.1007/s00464-013-3176-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-013-3176-0