Abstract
Background
Several factors may affect volume and dimensions of the working space in laparoscopic surgery. The precise impact of these factors has not been well studied. In a porcine model, we used computed tomographic (CT) scanning for measuring working space volume and distances. In a first series of experiments, we studied the relationship between intra-abdominal pressure (IAP) and working space.
Methods
Eleven 20 kg pigs were studied under standardized anesthesia and volume-controlled ventilation. Cardiorespiratory parameters were monitored continuously, and blood gas samples were taken at different IAP levels. Respiratory rate was increased when ETCO2 exceeded 7 kPa. Breath-hold CT scans were made at IAP levels of 0, 5, 10, and 15 mmHg. Insufflator volumes were compared to CT-measured volumes. Maximum dimensions of pneumoperitoneum were measured on reconstructed CT images.
Results
Respiratory rate had to be increased in three animals. Mild hypercapnia and acidosis occurred at 15 mmHg IAP. Peak inspiratory pressure rose significantly at 10 and 15 mmHg. CT-measured volume increased relatively by 93 % from 5 to 10 mmHg IAP and by 19 % from 10 to 15 mmHg IAP. Comparing CT volumes to insufflator volumes gave a bias of 76 mL. The limits of agreement were −0.31 to +0.47, a range of 790 mL. The internal anteroposterior diameter increased by 18 % by increasing IAP from 5 to 10 mmHg and by 5 % by increasing IAP from 10 to 15 mmHg. At 15 mmHg, the total relative increase of the pubis–diaphragm distance was only 6 %. Abdominal width did not increase.
Conclusions
CT allows for precise calculation of the actual CO2 pneumoperitoneum volume, whereas the volume of CO2 released by the insufflator does not. Increasing IAP up to 10 mmHg achieved most gain in volume and in internal anteroposterior diameter. At an IAP of 10 mmHg, higher peak inspiratory pressure was significantly elevated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Minimal access surgery is used for a wide variety of surgical conditions in both adults and children [1, 2]. An essential precondition for this approach is sufficient working space providing good view and ease of instrument handling [3–5]. In laparoscopy, this is mostly achieved by intraperitoneal insufflation of CO2 [6]. Within limits, the higher the pressure, the more working space is generated. On the other hand, the CO2 pneumoperitoneum has adverse effects. Absorption of CO2 may cause acidosis, and the increased intra-abdominal pressure (IAP) interferes with cardiorespiratory function and regional perfusion [6–14]. In pediatric minimal access surgery, the working space is inversely related to the patient’s size and thus also to age. It is tempting to increase intraperitoneal insufflation pressure, but the adverse consequences of CO2 insufflation may be more evident in children [10, 15]. Therefore, a delicate balance between intra-abdominal CO2 insufflation pressure and ventilatory settings is needed for patient safety on the one hand and having a good working space on the other [16–22]. Because there are few experimental data in the literature addressing this delicate balance, we decided to study it in a porcine model.
Working space in laparoscopy is determined by three types of factors: patient related, pneumoperitoneum related, and anesthesiology related (Table 1).
In a first series of experiments, we addressed the relationship between one of the pneumoperitoneum-related factors—CO2 insufflation pressure—and working space in a porcine model with volume-controlled ventilation. The effects of different CO2 insufflation pressures on pneumoperitoneum volume and intra-abdominal linear dimensions were investigated by computed tomography (CT).
Materials and methods
The institutional animal ethics committee granted approval for the experiments. Female pigs weighing approximately 20 kg were used. The animals were not fasted. On the day of the experiment, they were given an intramuscular injection of 1 mg/kg midazolam and 30 mg/kg ketamine. Spontaneous breathing was maintained. They were then transferred from the animal housing facility to the laboratory, where a cannula was placed in the auricular vein. A continuous intravenous infusion of 6–8 mg/kg/h propofol and 4 μg/kg/h sufentanil was initiated. No neuromuscular blocking agents were used throughout the experiments. Next, tracheotomy was performed through a midline cervical incision, and volume-controlled ventilation (EvitaXL; Dräger, Lübeck, Germany) was started with an air–oxygen mixture (FiO2 40 %), I:E ratio at 1:2, a tidal volume of 10 mL/kg, and a rate of 40/min. Positive end expiratory pressure (PEEP) was set at 5 cm H2O. End-tidal CO2 (ETCO2) was monitored on the ventilator. Tidal volume was kept constant; peak inspiratory pressures (PIP) were recorded during the experiment. Respiration rate was adjusted to maintain ETCO2 within a range of 3.5–7 kPa.
A nasal temperature probe was placed and normothermia (38–40 °C) was maintained with the use of an electric heating blanket (Inventum, Veenendaal, The Netherlands). Cardiac monitoring was initiated at this point with a three-lead ECG. An intra-arterial line (Arrow 20 G; Arrow, Reading, PA, USA) was placed in the right carotid artery for continuous blood pressure measurement and sampling of blood for hematocrit and blood gas analysis. A venous line (Percutaneous Sheath Introducer Set 8.5F; Arrow) was placed in the right internal jugular vein via a separate low-cervical incision.
A supraumbilical midline abdominal trocar was placed after insufflation of the abdominal cavity with CO2 through a Veress needle to an IAP of 5 mmHg. Insufflation was by means of an electronic insufflator (Endoflator, Storz, Tuttlingen, Germany). To prevent leakage of CO2 and trocar-site bleeding, a radially expanding trocar (VersaStep 5 mm; Covidien, Dublin, Ireland) was used [23, 24]. Correct intra-abdominal placement was verified by laparoscopy (Storz Telepack and 5 mm 30° telescope). The abdomen was then desufflated by opening the CO2 inlet of the trocar. A 30 min infusion of 500 mL of colloid (Voluven; Fresenius Kabi, Halden, Norway) was provided, followed by continuous infusion of 10 mL/kg/h of isotonic saline. When hemodynamic and respiratory parameters were stable, the pig was transported to a CT scanner (Definition Flash Dual Source; Siemens, Erlangen, Germany). The electronic CO2 insufflator was attached to the abdominal trocar after the pig was installed on the scanning tray. Thorax and abdomen were scanned at IAPs of 0, 5, 10, and 15 mmHg. To minimize respiratory motion artifacts, scans were made during expiratory arrests while maintaining PEEP at 5 cm H2O. Scanning duration for a CT run was approximately 5 s. At each new pressure level, further scanning was paused until PIP and ETCO2 had stabilized, which always was within 5 min. Blood gas samples were taken directly after scanning before proceeding to the next pressure level, and values of cardiorespiratory parameters were recorded at these points. Pigs were humanely killed after scanning.
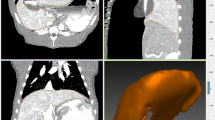
Three different measures of working space were analyzed: linear dimensions as measured on CT (Fig. 1) in a sagittal midline plane; maximum internal abdominal anteroposterior (AP) diameter from the front of the vertebral column to the anterior peritoneal lining; maximum distance between the upper border of the pubic symphysis and the highest diaphragmatic peritoneal lining; in a transverse and coronal plane; maximal internal diameter of the abdomen between the lateral peritoneal linings; and volume of the free intra-abdominal CO2 as measured on CT. Intra-abdominal volumes were calculated with the Syngo 3D volume module of a Siemens Navigator workstation using a data set of 5 mm slices. Free CO2 in the abdomen on transverse slices was detected semiautomatically after appropriate thresholds were defined. Slices could be integrated to a total volume of free intra-abdominal CO2. All volumes were visually checked for inadvertent inclusion of intraluminal gas in the bowel, which has the same CT density as CO2. Volume of insufflated CO2 as given by the insufflator (Endoflator, Storz).
All data were analyzed by SPSS software for Windows, version 16 (SPSS, Chicago, IL, USA). The increase in linear dimensions of abdominal working space with increasing IAP was measured on reconstructed CT images. The volume indicated by the electronic insufflator was compared to the volume measured on CT. Agreement between these volume measurements was visualized in a Bland–Altman plot [25]. The significance of changes in cardiorespiratory measurements at the different IAPs was calculated with paired t tests. A p value of less than 0.05 was taken to signify a statistical difference.
Results
Twelve pigs entered the study. Mean body weight was 22.8 (range, 19.2–25.2) kg. However, one pig died during surgical preparation; data of 11 pigs were analyzed.
Cardiorespiratory monitoring data including blood gas analysis are presented in Table 2. As described in the anesthesia protocol, respiratory rate was adjusted to compensate for hypercapnia during the experiments (ETCO2 > 7 kPa). This was done in three pigs: in two with an increase of five breaths per minute, and in one with a two-stepped increase to a total of 10 breaths/minute. Table 2 shows that the PIP significantly increased to a maximum of 28 cm H2O when IAP was raised to 15 mmHg. Mild hypercapnia (ETCO2 6.49 kPa) occurred with this increased IAP.
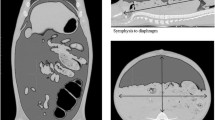
Regarding the linear dimensions on CT (Fig. 2), the mean internal AP diameter increased by 3.2 cm (from 8.8 to 12 cm) when IAP was raised from 0 to 5 mmHg; a relative increase of 36 %. This diameter increased by another 2.2 cm (to 14.2 cm) when IAP was raised from 5 to 10 mmHg, a relative increase of 18 %. At the final step from 10 to 15 mmHg, the mean AP diameter increased by 0.8 cm, a relative increase of 5 %.
In contrast to the AP diameter, the transverse diameter did not significantly increase with increasing pressure. It even slightly, but nonsignificantly (p = 0.154), decreased at the maximum IAP of 15 mmHg.
The mean distance between the pubic symphysis and the highest point of the peritoneal lining of the diaphragm increased a total of 2.2 cm (6 % relative increase) with increasing IAP. This increase was statistically significant (p < 0.01).
A combined pressure–volume curve for all 11 pigs is depicted in Fig. 3. It shows the CT-measured volumes of intra-abdominal CO2 at the predefined IAPs. Mean volume of insufflated CO2 at the lowest pressure of 5 mmHg was 1.271 L. It increased to a mean of 2.459 L at 10 mmHg, a relative increase of 93 %. At 15 mmHg, it increased further to a mean of 2.919 L, a relative increase of 19 %.
Figure 4 shows a Bland–Altman plot in which the volumes of intra-abdominal CO2 measured on CT are plotted against the volumes as indicated on the electronic insufflator. The bias is 0.076 L, indicating that CT measures a somewhat larger volume than the insufflator. The limits of agreement are −0.31 to +0.47, a range of 0.79 L.
Discussion
“The higher the pressure, the better the view” is a remark heard from laparoscopic surgeons [26]. This axiom is only true within limits. Pressure increments at higher pressures will cause less gain in working space than increments at lower IAP levels. This can be explained by the mechanical properties of the abdominal wall. The abdominal wall becomes progressively less compliant at higher levels of deformation (stretch) [27]. Song et al. [28, 29] described anisotropic mechanical properties of the abdominal wall on the basis of the orientation of stiff connective tissue fibers. As explained in physiology textbooks, muscle-containing tissues have active and passive states where muscle tone greatly influences mechanical properties [30]. The stretching of muscles also influences the maximum force their sarcomeres can generate [31]. This makes the abdominal wall a nonlinear, anisotropic, dynamic, and difficult-to-describe mechanical entity. Moreover, increasing IAP negatively affects cardiorespiratory function and tissue oxygenation, and may result in more postoperative pain [6–14].
With this study, we aimed to gain a more precise insight into the effects of increasing IAP on working space distances and volume against the background of cardiorespiratory function. We created a stable porcine model using an anesthesia protocol that allowed for an ETCO2 limit of 7 kPa (permissive hypercapnia). Levels of ETCO2 rose with increasing IAP. Ventilator rate needed to be adjusted in three animals only in order to keep ETCO2 within the desired limit. A statistically significant rise in PIP occurred when the insufflation pressure was raised to 10 and 15 mmHg. Such high pressures may damage the lungs [32, 33]. A pneumoperitoneum pressure of 15 mmHg is considered high for a juvenile 20 kg pig [34] and is considered to be the upper limit in laparoscopy in adult humans as well [6, 19].
CT revealed a nonlinear increase of the abdominal volume with increasing pressure (Fig. 3). The pressure rise to 10 mmHg achieved the most gain in working space; the next step to 15 mmHg achieved much smaller gain.
We found a marginal but statistically significant cranial displacement of the diaphragm with increasing IAP. The limited displacement is due to the volume-controlled ventilation with PEEP. The pneumoperitoneum transverse diameter did not change significantly. Therefore, the internal AP diameter was the only dimension substantially influenced by the IAP created by CO2 insufflation. For the best result, this should be taken into account when positioning the patient and presenting the area of surgical interest [35, 36].
Use of the volume of CO2 released by the insufflator as the only indicator of the amount of CO2 that is in the abdomen results in errors due to gas leakage, absorption of gas, and effects of temperature and compressibility. In contrast, multiplanar CT analysis of the working space is a reliable way to measure CO2 peritoneum and its linear dimensions because it clearly depicts the boundaries of the actual working space (Fig. 1) [37].
As shown in Fig. 4, the limits of agreement between the volumes measured on CT and the volumes indicated by the insufflator span a large range of 0.79 L. This inaccuracy is comparable to the size of the effect of interventions aimed at increasing working space.
Determining an optimal relationship between working space and homeostasis during laparoscopic procedures is not easy [16–22]. In contrast to our experiments, complex minimal access surgery in humans can take several hours, adding to the negative effects of the CO2 pneumoperitoneum. Avoiding high insufflation pressure will counteract the negative influences of CO2 insufflation [6, 14, 19, 26].
Exact measurement of the working space against the background of cardiorespiratory monitoring is imperative when studying how certain factors can influence the working space. CT scanning allows for such an exact measurement. Pressure of pneumoperitoneum was the first factor we investigated. Experiments on the effects of other factors mentioned in Table 1 are in preparation. The findings can help improve surgical and anesthesia management in minimal access surgery.
References
Scott-Conner CEH (1999) The SAGES manual: fundamentals of laparoscopy and GI endoscopy. Springer, New York
Bax K, Georgeson KE, Rothenberg SS, Valla JS, Yeung CK (2008) Endoscopic surgery in infants and children. Springer, Berlin
Emam TA, Hanna GB, Kimber C, Dunkley P, Cuschieri A (2000) Effect of intracorporeal–extracorporeal instrument length ratio on endoscopic task performance and surgeon movements. Arch Surg 135(1):62–65
Frede T, Stock C, Renner C, Budair Z, Abdel-Salam Y, Rassweiler J (1999) Geometry of laparoscopic suturing and knotting techniques. J Endourol 13(3):191–198
Hanna GB, Shimi S, Cuschieri A (1997) Influence of direction of view, target-to-endoscope distance and manipulation angle on endoscopic knot tying. Br J Surg 84(10):1460–1464
Neudecker J, Sauerland S, Neugebauer E, Bergamaschi R, Bonjer HJ, Cuschieri A, Fuchs KH, Jacobi Ch, Jansen FW, Koivusalo AM, Lacy A, McMahon MJ, Millat B, Schwenk W (2002) The European association for endoscopic surgery clinical practice guideline on the pneumoperitoneum for laparoscopic surgery. Surg Endosc 16(7):1121–1143
Ishizaki Y, Bandai Y, Shimomura K, Abe H, Ohtomo Y, Idezuki Y (1993) Changes in splanchnic blood flow and cardiovascular effects following peritoneal insufflation of carbon dioxide. Surg Endosc 7(5):420–423
Blobner M, Bogdanski R, Kochs E, Henke J, Findeis A, Jelen-Esselborn S (1998) Effects of intraabdominally insufflated carbon dioxide and elevated intraabdominal pressure on splanchnic circulation: an experimental study in pigs. Anesthesiology 89(2):475–482
Jakimowicz J, Stultiens G, Smulders F (1998) Laparoscopic insufflation of the abdomen reduces portal venous flow. Surg Endosc 12(2):129–132
Ure BM, Suempelmann R, Metzelder MM, Kuebler J (2007) Physiological responses to endoscopic surgery in children. Semin Pediatr Surg 16(4):217–223
Koivusalo AM, Lindgren L (1999) Respiratory mechanics during laparoscopic cholecystectomy. Anesth Analg 89(3):800
Dexter SP, Vucevic M, Gibson J, McMahon MJ (1999) Hemodynamic consequences of high- and low-pressure capnoperitoneum during laparoscopic cholecystectomy. Surg Endosc 13(4):376–381
Reintam Blaser A, Bloechlinger S (2010) Should we be worried about disturbed sympathovagal balance during laparoscopic cholecystectomy? Minerva Anestesiol 76(11):876–878
Rishimani AS, Gautam SC (1996) Hemodynamic and respiratory changes during laparoscopic cholecystectomy with high and reduced intraabdominal pressure. Surg Laparosc Endosc 6(3):201–204
Tobias JD (2002) Anaesthesia for minimally invasive surgery in children. Best Pract Res Clin Anaesthesiol 16(1):115–130
Cadi P, Guenoun T, Journois D, Chevallier JM, Diehl JL, Safran D (2008) Pressure-controlled ventilation improves oxygenation during laparoscopic obesity surgery compared with volume-controlled ventilation. Br J Anaesth 100(5):709–716
Andersson L, Lagerstrand L, Thörne A, Sollevi A, Brodin LA, Odeberg-Wernerman S (2002) Effect of CO2 pneumoperitoneum on ventilation–perfusion relationships during laparoscopic cholecystectomy. Acta Anaesthesiol Scand 46(5):552–560
Balick-Weber CC, Nicolas P, Hedreville-Montout M, Blanchet P, Stéphan F (2007) Respiratory and haemodynamic effects of volume-controlled vs pressure-controlled ventilation during laparoscopy: a cross-over study with echocardiographic assessment. Br J Anaesth 99(3):429–435
Gurusamy KS, Samraj K, Davidson BR (2009) Low pressure versus standard pressure pneumoperitoneum in laparoscopic cholecystectomy. Cochrane Database Syst Rev (2), Art. No. CD006930. doi:10.1002/14651858.CD006930.pub2
Gerges FJ, Kanazi GE, Jabbour-Khoury SI (2006) Anesthesia for laparoscopy: a review. J Clin Anesth 18(1):67–78
Maracajá-Neto LF, Verçosa N, Roncally AC, Giannella A, Bozza FA, Lessa MA (2009) Beneficial effects of high positive end-expiratory pressure in lung respiratory mechanics during laparoscopic surgery. Acta Anaesthesiol Scand 53(2):210–217
Vegfors M, Engborg L, Gupta A, Lennmarken C (1994) Changes in end-tidal carbon dioxide during gynecologic laparoscopy: spontaneous versus controlled ventilation. J Clin Anesth 6(3):199–203
Ahmad G, O’Flynn H, Duffy JMN, Phillips K, Watson A (2012) Laparoscopic entry techniques. Cochrane Database Syst Rev (2), Art. No. CD006583. doi:10.1002/14651858.CD006583.pub3
Bhoyrul S, Payne J, Steffes B, Swanstrom L, Way LW (2000) A randomized prospective study of radially expanding trocars in laparoscopic surgery. J Gastrointest Surg 4(4):392–397
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1(8476):307–310
Yasir M, Mehta KS, Banday VH, Aiman A, Masood I, Iqbal B (2012) Evaluation of post operative shoulder tip pain in low pressure versus standard pressure pneumoperitoneum during laparoscopic cholecystectomy. Surgeon 10(2):71–74
Fung YC (1993) Biomechanics: mechanical properties of living tissues, 2nd edn. Springer, New York
Song C, Alijani A, Frank T, Hanna G, Cuschieri A (2006) Elasticity of the living abdominal wall in laparoscopic surgery. J Biomech 39(3):587–591
Song C, Alijani A, Frank T, Hanna GB, Cuschieri A (2006) Mechanical properties of the human abdominal wall measured in vivo during insufflation for laparoscopic surgery. Surg Endosc 20(6):987–990
Berne RM, Levy MN (1988) Physiology, 2nd edn. Mosby, St. Louis
Gordon AM, Huxley AF, Julian FJ (1966) The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol 184(1):170–192
Gattinoni L, Protti A, Caironi P, Carlesso E (2010) Ventilator-induced lung injury: the anatomical and physiological framework. Crit Care Med 38(10 suppl):S539–S548
Slinger P (2008) Perioperative lung injury. Best Pract Res Clin Anaesthesiol 22(1):177–191
Gudmundsson FF, Heltne JK (2004) Respiratory changes during prolonged increased intra-abdominal pressure in pigs. Acta Anaesthesiol Scand 48(4):463–468
Bannenberg JJ, Meijer DW, Klopper PJ (1994) The prone position. Using gravity for a clear view. Surg Endosc 8(9):1115–1116
Hashimoto M, Matsuda M, Watanabe G (2008) Simple method of laparoscopic splenectomy. Surg Endosc 22(11):2524–2526
Cai W, Tabbara M, Takata N, Yoshida H, Harris GJ, Novelline RA, de Moya M (2009) MDCT for automated detection and measurement of pneumothoraces in trauma patients. Am J Roentgenol 192(3):830–836
Acknowledgments
We thank P. Specht for assistance in the experiments and M. Dijkshoorn for CT scanning.
Disclosures
John Vlot, Rene Wijnen, Robert Jan Stolker, and Klaas Bax have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vlot, J., Wijnen, R., Stolker, R.J. et al. Optimizing working space in porcine laparoscopy: CT measurement of the effects of intra-abdominal pressure. Surg Endosc 27, 1668–1673 (2013). https://doi.org/10.1007/s00464-012-2654-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-012-2654-0