Abstract
Introduction
Bacterial contamination from viscerotomy is a barrier to natural orifice translumenal endoscopic surgery (NOTES). The aim of this survival study is to evaluate pure (totally) transvaginal NOTES bacterial contamination compared with laparoscopy in pigs.
Methods
Twelve adult female pigs underwent peritoneoscopy with liver and peritoneal biopsies, using either laparoscopy (Glap, six animals) or pure transvaginal (GNOTES) access, and were maintained alive for 7 days. In all animals, blood cultures were taken at baseline, and after 24 h and 7 days postoperatively. Swab cultures from vagina (GNOTES) and skin (Glap) were obtained pre- and post-antisepsis. Peritoneal fluid culture was obtained at necropsy. For statistical analysis, Glap and GNOTES were compared for presence of positive bacterial cultures (qualitative bacterial analysis) using Fisher’s test, with level of significance set at p < 0.05.
Results
All animals had good postoperative outcome. One animal had transient perioperative bleeding from a transvaginal access. Two animals in Glap and one in GNOTES had positive blood cultures after the procedure. All animals from GNOTES and Glap presented with mixed flora pre-antisepsis. After antisepsis, one animal (GNOTES) presented with a positive vaginal swab culture (a single bacterial strain was identified). There was no positive skin swab culture in Glap. There were no signs of intra-abdominal infection at necropsy. In two animals, one from Glap and another from GNOTES, intra-abdominal culture was positive for Corynebacterium spp. and Escherichia coli, respectively. There was no correlation between the bacterial flora found at the access site and in the peritoneal cultures.
Conclusions
Pure transvaginal peritoneoscopy with liver and peritoneal biopsy in swine is feasible and associated with bacterial contamination comparable to laparoscopy. Peritoneal bacterial contamination was clinically insignificant after 1 week postoperatively. Preoperative antisepsis provided significant reduction of bacterial load prior to transvaginal and laparoscopic procedures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Natural orifice translumenal endoscopic surgery (NOTES) is a surgical technique in which access is usually gained by transgressing the visceral wall. Because of its avoidance of skin incisions, one of the main theoretical advantages of NOTES is an appealing cosmetic advantage and less postoperative pain due to preservation of parietal somatic nerves that are injured during skin, fascia, and muscular parietal transgression during laparoscopic or open surgery. Currently, NOTES procedures use mainly transgastric and transvaginal routes and have been proven feasible for performing several procedures including diagnostic peritoneoscopy, liver biopsies, tubal ligation, appendectomy, and cholecystectomy, among others [1].
Despite significant interest in NOTES, there are still concerns regarding its safety. Prevention of infection and secure closure of the entry point of NOTES procedures remain important issues to be addressed [1]. Although there are clinical studies evaluating postoperative outcome following transvaginal hysterectomy [2, 3] and transvaginal NOTES cholecystectomy [4, 5], few experimental studies have accessed the risk of bacterial contamination from transvaginal access [6, 7]. To our knowledge, none of them have compared transvaginal NOTES with the standard of care, laparoscopy. We hypothesize that transvaginal NOTES is not associated with increased bacterial contamination compared with laparoscopy for performing peritoneoscopy with liver and peritoneal biopsy.
The aim of this study is to compare pure (or totally) transvaginal diagnostic peritoneoscopy with liver and peritoneal biopsy versus conventional laparoscopy in terms of bacterial contamination. Postoperative outcome and bacterial cultures from blood, access sites, and peritoneal fluid were analyzed in the context of a 1-week survival period.
Materials and methods
Twelve female cross-bred Yorkshire swine (Sus scrofus domesticus, Mammalia) from Granja Bela Vista (Campo Magro, Paraná, Brazil), weighting 40–45 kg, underwent either transvaginal or laparoscopic diagnostic peritoneoscopy with liver and peritoneal biopsy. All procedures were approved by the local animal ethics committee. The animals were housed and the procedures were performed at the Universidade Positivo experimental animal facilities.
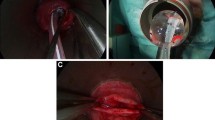
For transvaginal NOTES procedures, a single-channel endoscope (GIF 140; Olympus, Tokyo, Japan) was used. Disposable endoscopic accessories such as needle-knife, biopsy forceps, and rat-toothed graspers (Olympus, Tokyo, Japan) were used for peritoneoscopy and liver biopsies. A 12-mm disposable laparoscopic trocar (Versaport; Covidien, Mansfield, MA) was used for obtaining transvaginal access. This trocar was then replaced by a customized NOTES trocar (Fig. 1) for performing the peritoneoscopy and biopsies. This trocar was designed for maintaining pneumoperitoneum and to avoid peritoneal contamination from multiple endoscope passings. For the laparoscopic procedures, a standard video laparoscope system (Karl Storz, Tuttlingen, Germany), including a 0° laparoscope, was used. Permanent laparoscopic surgical instruments including graspers, scissors, dissectors, and trocars (EDLO, Canoas, Brazil) were used for laparoscopic peritoneoscopy and liver biopsies. A laparoscopic insufflator was used for maintaining the abdominal pressure (Electronic Endoflator; Karl Storz, Tuttlingen, Germany) in all procedures. All procedures were performed using skin antisepsis with aqueous povidone iodine (Betadine; Purdue, Stanford, USA).
High-level disinfection of the permanent laparoscopic material and the flexible endoscope included mechanical cleansing and 30-min immersion in hydrogen peroxide (Sekusept Aktiv®; Ecolab GmbH, Minnesota, USA), as recommended by the manufacturer.
Surgical procedures
After a period of acclimation, the animals were fasted from solid food (12 h prior to procedure). Water was allowed ad libitum until 6 h prior to the procedure. All animals were maintained alive for 7 days postoperatively.
Animals were divided into two groups: laparoscopy group (Glap, six animals) and NOTES group (GNOTES, six animals). All animals underwent standardized exploratory peritoneoscopy with liver and peritoneal biopsies [8].
General anesthesia was carried out using pre-anesthetic induction with intramuscular ketamine 14 mg/kg, xylazine 2 mg/kg, and acepromazine 0.4 mg/kg. Thiopental 10 mg/kg was used intravenously. For maintenance, intravenous infusion of propofol 1–3 mg/kg and inhalatory isoflurane 2 % was used. Lactate Ringer infusion at 10 ml/kg/h was used for hydration. Prophylactic antibiotics were administered intravenously in all animals (ampicillin 1 g + sulbactam 500 mg). After gaining access to the abdominal cavity, CO2 pneumoperitoneum was maintained at 10 mmHg. All surgical procedures were planned to have similar duration between groups. The planned average time for all procedures was 40 min. Timing was determined based on previous pilot transvaginal NOTES procedures [8].
Laparoscopic procedures
For the laparoscopic procedures, four trocars (two of 10 mm, two of 5 mm) were placed in triangulation at the left and right abdominal flanks. The laparoscope was inserted at the umbilicus, and after thorough abdominal inspection (right and left, upper and lower quadrants), two liver (right and left lobes) and four peritoneal (one for each quadrant) biopsies were obtained.
Pure transvaginal NOTES procedures
We designed a pure (totally) NOTES procedure to avoid the influence of skin incisions on bacterial contamination of the peritoneal cavity. Transvaginal access was undertaken by blind trocar insertion at the anterior vaginal wall. This site was defined as being 1 cm proximal to the opening of the urethra based on prior anatomic dissections (Fig. 2). For this procedure, we developed and chose the anterior transvaginal access approach because the urethra opening of the female pig is located deep in the vagina, allowing a 2–3-cm proximal vaginal wall space for trocar placement [8]. By using this approach, the bladder and rectum are spared from injuries, allowing safe blunt trocar insertion. However, care must be taken to avoid sliding the trocar into the urethra while attempting vaginal puncture. High-level disinfection of endoscopes was obtained using 30-min immersion in peroxide hydrogen, an accepted practice for gastrointestinal endoscopy [9]. For NOTES procedures, sterilization of endoscopes is recommended but not yet validated [9]. Therefore, bacterial contamination is a possible complication arising from use of high-level disinfected endoscopes; however, its determination lies beyond the scope of this study.
The trocar was initially inserted transvaginally, aiming at the inferior border of pubic bone. Then, it was progressively advanced at 45° until reaching a bone-like structure (inferior border of the pubic bone). After passing the pubic bone, the trocar was further inclined up to 60° and advanced to create the transvaginal access by puncturing the anterior vaginal wall. After gaining access, the flexible endoscope was advanced, reaching the retroperitoneal cavity, which was further bluntly dissected to gain peritoneal access. The laparoscopic trocar was then replaced by the customized NOTES trocar for establishment and maintenance of pneumoperitoneum. After thorough abdominal inspection, two liver (right and left lobes) and four peritoneal (one for each quadrant) biopsies were undertaken. All surgical specimens were placed into formalin and sent for hematoxylin and eosin (H&E) histopathological analysis in blind fashion. Skin closure was carried out using nonabsorbable subcutaneous sutures (Mononylon 3.0; Ethicon, São José dos Campos, Brasil). The transvaginal access was not closed. Further description of surgical procedures for these experiments can be found elsewhere [8].
Bacterial contamination assessment
Vaginal cultures were obtained from vaginal swabs performed pre- and post-antisepsis (Fig. 3). For the laparoscopy group, swab from the abdominal skin (at trocar sites) was obtained pre- and post-antisepsis. Skin and vaginal antisepsis was performed by vaginal embrocation with aqueous povidone iodine (Betadine; Purdue, Stanford, USA). Blood cultures were obtained at anesthesia induction (baseline), after 24 h postoperatively, and at euthanasia (7th day postoperatively) using skin disinfection to avoid contamination. Samples of peritoneal fluid from upper abdomen (right upper quadrant) and pelvis were obtained at necropsy and sent for culture. All samples from vagina, skin, and peritoneum were stored in enriched culture media (CRAL; Cotia, Brazil) and sent for analysis at LACEN laboratories (Curitiba, Brazil). All material obtained from culture media was incubated in blood, chocolate, and MacConkey agar plates and brain–heart infusion (BHI) broth for aerobes and anaerobes. Culture plates were read after 24 and 48 h. If bacterial growth was observed, proper identification was performed using Gram and specific bacterial identification analysis.
Postoperative evaluation
During the surgical procedures, all animals were monitored for cardiovascular, oxygenation, and CO2 concentration events. Complications such as bleeding or inadvertent adjacent organ injuries were recorded. Postoperatively, all animals received water and swine chow ad libitum after anesthesia recovery. All animals were clinically evaluated daily for presence of complications and euthanized at 7 days. Euthanasia was undertaken using a lethal dose of thiopental 2.5 % (20 mg/kg), and animals had their weight measured. For necropsy, midline laparotomy was performed for assessment of complications, such as infection and adhesions. The transvaginal access site was also inspected for complications. Samples of peritoneal fluid from upper abdomen and pelvis were sent for culture and bacterial analysis.
Statistical analysis
Data analysis was undertaken for presence of bacterial contamination (qualitative bacterial analysis of vaginal and skin swabs, peritoneal fluid, and blood cultures). The exact Fisher test was used for paired analysis between Glap and GNOTES groups, using SAS software (version 9.1.3; SAS Institute, Cary, NC, USA; 2002–2003). Results are expressed as mean (standard deviation, SD). A p value of 0.05 was adopted for significance.
Results
Overall, all animals survived and thrived well during the postoperative period. The duration of anesthesia and surgical procedures was 41 min on average [Glap 39.8 (11.2) min; GNOTES 42.8 (9.8) min]. One animal had transient bleeding while attempting to perform a transvaginal access, which did not influence the clinical outcome. None of the other animals had complications. All tissue from liver and peritoneal biopsies was subjected to histopathological analysis. Further information on perioperative parameters of these animals is described elsewhere [8].
Bacterial contamination assessment
Blood cultures
Blood cultures were all negative at baseline. However, 24 h after the procedure one animal from Glap presented with blood culture positive for Staphylococcus aureus. On the 7th postoperative day, blood culture was positive for Escherichia coli in one animal from Glap and in one animal for S. aureus for GNOTES (Table 1). There were no statistically significant differences between groups.
Cultures from access sites and peritoneal fluid
All animals presented with positive mixed flora cultures from both skin and vaginal access sites before antisepsis. After antisepsis with povidone iodine, one animal from GNOTES had access-site (vaginal) culture positive for Streptococcus dysgalactiae subsp. equisimilis). On the 7th postoperative day, two animals had positive cultures at the pelvic region, one from Glap and another from GNOTES; the isolated pathogens were Corynebacterium spp. and E. coli, respectively (Table 2). There were no statistically significant differences between groups (p = 1.000). Peritoneal fluid cultures from the upper quadrant were negative in all animals. The bacteria isolated from the peritoneum in these animals were not the same as found in their respective access sites (no proven cross-contamination).
Discussion
Bacterial contamination is of paramount importance for postoperative infection and ultimately NOTES outcome. Despite many efforts to develop novel techniques and devices to enable NOTES procedures, there are few studies assessing the risk of cross-contamination and bacterial translocation during transvaginal NOTES procedures [6, 7]. To our knowledge, none of them have compared transvaginal access with standard laparoscopy. Preventive measures to avoid infection used for current transvaginal NOTES are in fact adapted from transvaginal hysterectomy and gynecological procedures [10]. This latter approach differs in many aspects from current transvaginal NOTES procedures, especially regarding the type of vaginal access (incision opening vs. puncture) and procedure type (restricted only to pelvis vs. distant organs). Also, transvaginal peritoneoscopy, a model for NOTES procedures, is comparatively a simpler and a short-duration procedure. In this study, we chose a pure (totally) transvaginal approach to represent a true NOTES procedure.
As contamination may arise directly from the access site, preoperative antisepsis of the access site should be considered an important step before creating translumenal access [11]. One of the main issues for NOTES procedures is to accomplish adequate antisepsis of the entire natural orifice route (down to viscerotomy), especially for the digestive tract or vaginal routes. There is some limitation on use of some current disinfecting agents, since alcohol-based solutions irritate the mucosa. Also, mechanical antisepsis with scrubbing is easily applicable to procedures involving the skin; however, it may not be applicable for some NOTES procedures. Mechanical antisepsis with scrubbing may in fact not be necessary for surgical procedures. In a randomized study on 234 patients, adequate skin antisepsis could be achieved using aqueous povidone iodine painting without the need for scrubbing [12]. For transvaginal procedures, vaginal embrocation with povidone iodine provides mechanical antisepsis of the access site [6], whereas for gastrointestinal NOTES procedures, topical disinfection relies only on lavage with antiseptics [11, 13]. In one randomized clinical study, chlorhexidine seemed superior to povidone iodine for vaginal antisepsis prior to transvaginal hysterectomy [14]. In the present study, preprocedural vaginal embrocation with povidone iodine was associated with significant reduction of bacterial flora load.
Transvaginal access has some advantages over other NOTES access (i.e., transgastric, transrectal) due to the virtual absence of risk for fistula, unless there is inadvertent transgression of another adjacent organ (i.e., rectum). Antisepsis can be especially challenging for large contaminated cavities such as hypo/achlorhydric stomach, e.g., in case of chronic use of proton pump inhibitors (PPIs) [15], and the colon. Chronic usage of PPI is associated with a higher rate of peritoneal bacterial contamination, which can potentially influence outcome. However, in an experimental study using pigs with usage of PPI, use of intravenous antibiotics in addition to topical antimicrobial lavage of mouth and stomach decreased the peritoneal bacterial load to almost zero, and this was associated with a significantly lower peritoneal infection rate compared with saline-only lavage. In another study involving 50 patients undergoing transgastric peritoneoscopy, previous use of PPI was documented in 17 and this did not lead to clinical significant infection [13]. In experimental studies comparing transgastric and transvaginal NOTES procedures in pigs, transvaginal NOTES was associated with less contamination [6] and infectious complications [7]. In the present study, peritoneal contamination was detected in two animals (one from laparoscopy and another from transvaginal NOTES) after 7 days, neither of which was associated with the bacterial flora found at baseline (pre-antisepsis). However, for the animal from the NOTES group, as the isolated bacteria is a specimen that usually belongs to swine vaginal flora, one could argue that the baseline culture (pre-antisepsis) could be falsely negative. Nevertheless, there were no signs of clinical infection in any of these animals.
Antibiotic prophylaxis seems to significantly reduce postoperative infection following transvaginal hysterectomy [16] and has been recommended as a standard of practice with high level of evidence (1A) [17]. A single dose of a first-generation cephalosporin should be administered 15–60 min prior to skin incision. No additional doses are recommended. If patients are allergic to cephalosporin, then clindamycin, erythromycin, or metronidazole should be used [17]. Bacterial translocation is another concern for NOTES procedures. In the present study, positive blood cultures were found after 24 h (one animal undergoing laparoscopy) and after 1 week (one animal undergoing laparoscopy and one animal undergoing NOTES). Both animals had blood culture positive for skin flora bacteria, which could be associated with contamination during sampling, despite careful sampling technique. None of these animals had clinical signs of infection. In the light of these findings, these results should be interpreted carefully.
Single or multiple passage of a contaminated endoscope into the body cavity may lead to cross-carriage of bacteria. Therefore, one important measure is to secure the point of entry during the entire endoscopic procedure to avoid the endoscope making contact with natural orifice route secretions. A proposed solution to this problem is use of a NOTES port or trocar, which is still under experimental investigation [18, 19]. These overtube-type ports, extending through the natural orifice (NO) route to the viscerotomy, would theoretically act as sterile conduits, maintaining stable and secure access and minimizing peritoneal contamination. In our series, with the use of a customized trocar, we hypothesize that this could have prevented further bacterial contamination. Further studies are warranted to confirm this hypothesis.
One limitation of the present study is the evaluation of the relationship between peritoneal contamination and clinical peritonitis in a short-duration survival period (1 week). Although we found peritoneal contamination in three animals, ongoing peritonitis seemed to be unlikely, since there were no signs of peritoneal fluid infection such as change in coloration or fibrin accumulation. All three bacterial isolates [S. dysgalactiae subsp. equisimilis, E. coli (2791), and Corynebacterium spp.] are usually found on skin and mucosal flora of swine and are harmless under normal circumstances, unless they harbor any virulent factor [20]. Clinical studies have demonstrated that bacterial contamination from transgastric procedures does not necessarily translate into clinical peritonitis [13].
In conclusion, in the present study we have demonstrated that pure (totally) transvaginal NOTES peritoneoscopy with liver and peritoneal biopsy is feasible in swine and comparable to laparoscopy in terms of bacterial contamination. Peritoneal bacterial contamination seems to occur from cross-carriage (coming from point of surgical access) and, if present, does not seem to lead to clinical infection. Preoperative antisepsis with vaginal embrocation together with antibiotic prophylaxis seems a reasonable and effective approach to avoid infection and should be recommended prior to transvaginal procedures. However, antibiotic prophylaxis and antisepsis regimen for some specific less invasive transvaginal procedures (i.e., transvaginal peritoneoscopy) could be a matter for further study. Bacterial translocation may also occur from NOTES and seems to be comparable to laparoscopy; however, contamination of blood samples (bacterial skin flora) in swine poses a challenge for its interpretation. Extrapolation of the results of this study to humans should be done with care, since technical and anatomic issues may interfere in cross-contamination, and different bacterial flora (including sexually transmitted bacteria) may interfere in outcomes. Further studies are needed to assess, prevent, and identify risk factors for bacterial contamination and infection in transvaginal NOTES procedures.
References
Rattner DW, Hawes R, Schwaitzberg S et al (2011) The Second SAGES/ASGE White Paper on natural orifice transluminal endoscopic surgery: 5 years of progress. Surg Endosc 25(8):2441–2448
Mäkinen J, Johansson J, Tomás C et al (2001) Morbidity of 10110 hysterectomies by type of approach. Hum Reprod 16(7):1473–1478
Meltomaa SS, Mäkinen JI, Taalikka MO et al (1999) One-year cohort of abdominal, vaginal, and laparoscopic hysterectomies: complications and subjective outcomes. J Am Coll Surg 189(4):389–396
Lehmann KS, Ritz JP, Wibmer A et al (2010) The German registry for natural orifice translumenal endoscopic surgery: report of the first 551 patients. Ann Surg 252(2):263–270
Zorron R, Palanivelu C, Galvão Neto MP et al (2010) International multicenter trial on clinical natural orifice surgery—NOTES IMTN study: preliminary results of 362 patients. Surg Innov 17(2):142–158
Yang QY, Zhang GY, Wang L et al (2011) Infection during transgastric and transvaginal natural orifice transluminal endoscopic surgery in a live porcine model. Chin Med J (Engl) 124(4):556–561
Lomanto D, Chua HC, Myat MM et al (2009) Microbiological contamination during transgastric and transvaginal endoscopic techniques. J Laparoendosc Adv Surg Technol A 19(4):465–469
Claus CM, Bonin EA, Torres MF, Campos AC, Cury AM, Coelho JC (2011) Liver and peritoneal biopsy by laparoscopy or notes in pigs: comparison of operative parameters and postoperative evolution. Rev Col Bras Cir 38(4):253–259
Spaun GO, Goers TA, Pierce RA et al (2010) Use of flexible endoscopes for NOTES: sterilization or high-level disinfection? Surg Endosc 24(7):1581–1588
Sodergren MH, Pucher P, Clark J et al (2011) Disinfection of the access orifice in NOTES: evaluation of the evidence base. Diagn Ther Endosc 2011:245175. doi:10.1155/2011/245175
Eickhoff A, Vetter S, von Renteln D et al (2010) Effectivity of current sterility methods for transgastric NOTES procedures: results of a randomized porcine study. Endoscopy 42(9):748–752
Ellenhorn JD, Smith DD, Schwarz RE et al (2005) Paint-only is equivalent to scrub-and-paint in preoperative preparation of abdominal surgery sites. J Am Coll Surg 201(5):737–741
Narula VK, Hazey JW, Renton DB et al (2008) Transgastric instrumentation and bacterial contamination of the peritoneal cavity. Surg Endosc 22(3):605–611
Culligan PJ, Kubik K, Murphy M, Blackwell L, Snyder J (2005) A randomized trial that compared povidone iodine and chlorhexidine as antiseptics for vaginal hysterectomy. Am J Obstet Gynecol 192(2):422–425
Ramamoorthy SL, Lee JK, Mintz Y et al (2010) The impact of proton-pump inhibitors on intraperitoneal sepsis: a word of caution for transgastric NOTES procedures. Surg Endosc 24(1):16–20
Löfgren M, Poromaa IS, Stjerndahl JH, Renström B (2004) Postoperative infections and antibiotic prophylaxis for hysterectomy in Sweden: a study by the Swedish National Register for Gynecologic Surgery. Acta Obstet Gynecol Scand 83(12):1202–1207
Van Eyk N, van Schalkwyk J, Infectious Diseases Committee (2012) Antibiotic prophylaxis in gynaecologic procedures. J Obstet Gynaecol Can 34(4):382–391
Hashiba K, Siqueira PR, Brasil HA et al (2010) Expandable gastric port for natural orifice translumenal endoscopic surgery. J Laparoendosc Adv Surg Technol A 20(7):623–625
Wilhelm D, Meining A, von Delius S et al (2007) An innovative, safe and sterile sigmoid access (ISSA) for NOTES. Endoscopy 39(5):401–406
Brandt CM, Spellerberg B (2009) Human infections due to Streptococcus dysgalactiae subspecies equisimilis. Clin Infect Dis 49(5):766–772
Acknowledgments
The authors would like to acknowledge Lyssandra Cravucov and Jordani Rodrigues for helping with collection of all samples used in the study. We would also like to acknowledge Sirlei Pereira and Vanderlei Muller for preparing and caring for the animals used in the study.
Disclosures
Drs. Eduardo Aimore Bonin, Christiano Marlo Paggi Claus, Antonio Carlos Ligocki Campos, and Marcelo de Paula Loureiro and Veterinarian Maria Fernanda Torres have no conflicts of interest or financial ties to disclose. Dr. Leandro Totti Cavazzola is an Advisory Board Member for Hernia for Bard and a Consultant for Hernia for Covidien; none of these relations has relevance for the present study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aimore Bonin, E., Claus, C.M.P., Torres, M.F. et al. Evaluation of bacterial contamination after “pure” (totally) transvaginal NOTES® diagnostic peritoneoscopy with biopsies in swine: a comparative study with laparoscopy. Surg Endosc 27, 421–427 (2013). https://doi.org/10.1007/s00464-012-2448-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-012-2448-4