Abstract
Background
A highly reliable and safe means of gastric closure for natural orifice transluminal endoscopic surgery (NOTES) has yet to be developed. The authors have previously described the self-approximating transluminal access technique (STAT) as a means for gastrotomy closure in transgastric surgery. It has yet to be determined whether biologic mesh can be utilized in facilitating gastrotomy closure via STAT. The aim of this study was to determine the feasibility of implanting an acellular porcine dermal matrix (LifeCell) into the STAT tunnel and investigate whether it will become incorporated into the submucosal plane of the STAT tunnel.
Methods
Five pigs underwent transgastric left uterine horn resection utilizing STAT. For closure, the acellular porcine dermal matrix was implanted within the submucosal plane, occluding the seromuscular incision. The mucosal incision was then closed over the matrix with endoscopically placed clips. Necropsy was performed after a 3 week survival period. Histopathological evaluation of the tunnel and matrix was performed.
Results
The matrix was successfully implanted in all five animals. Average OR time was 151 ± 68 min. Average time to anchor and embed the matrix within the tunnel was 4 ± 1 and 9 ± 12 min, respectively. There was one duodenal perforation related to a balloon occlusion device. Postoperative course was unremarkable; the average weight gain at 3 weeks was 22 ± 5 lbs. On necropsy, one animal had some protrusion of the matrix at the serotomy, with adhesions to small bowel and liver. Histopathology revealed one clinically insignificant microabscess but otherwise demonstrated local inflammation and fibrovascular ingrowth into the matrix.
Conclusions
The porcine dermal matrix can be successfully implanted within the gastric submucosal plane and evidence of incorporation into the gastric wall by 3 weeks was demonstrated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Bioprosthetic meshes have been utilized in complex hernia repairs where the likelihood of contamination in the field is high [1]. These biologic meshes are cadaveric or animal-derived dermal matrices in which the epidermis has been separated and the antigenic cellular components have been removed [2, 3]. They are thought to cause less infectious complications than synthetic meshes through their ability to become re-vascularized and therefore promote clearance of bacteria and prevent infections [4]. Various applications of their use have been reported, including burns [5], breast reconstruction [6], dural replacement [7], bladder augmentation [8], and periosteum replacement [9]. These features and their broad utility may make bioprosthetic meshes useful in natural orifice transluminal endoscopic surgery (NOTES).
A fundamental challenge before the clinical application of NOTES is a safe and reliable abdominal access and closure [10]. Investigators have reported intra-abdominal abscesses [11] and bacterial peritonitis [12] secondary to incomplete gastrotomy closure and poor outcomes with colotomy closure [13]. To date no consensus on the most appropriate closure technique has been reached. Prior investigations by our group have demonstrated the self-approximating transluminal access technique (STAT) as a safe, reliable, and reproducible method to access the peritoneum and it provides a secure means of gastrotomy closure for peroral transgastric NOTES procedures [14–16]. STAT essentially is a submucosal tunneling technique that requires dissection of an intramural tunnel within the gastric wall. One of the advantages of STAT is its self-approximating nature, rendering a secure closure with minimal clips to close the mucosal incision. To date, we have experienced a zero gastrotomy site leak rate [17]. However, if the integrity of the submucosal tunnel should become compromised due to perforation or a tear and additional closure becomes necessary; several techniques such as endoscopic clips and tissue adhesive within the tunnel potentially could be used. Here we investigate the use of a novel biomaterial for the purpose of fortifying a STAT access site that may lend itself to other applications of mucosal or transluminal repair as well.
In this study an acellular porcine dermal matrix (APDM) was used to determine the feasibility of implanting the matrix into the gastric STAT tunnel as well as determine its properties with regard to gastrotomy closure in a porcine model. We hypothesize that once introduced into the intramural tunnel, the APDM will become incorporated into the healing submucosal layer of the stomach wall and improve the inherent self-sealing property of the STAT tunnel.
Materials and methods
Animals
Five female domestic pigs (Sus scrofus domesticus) with a mean weight of 28 kg were utilized in this study. All animals were housed in the animal research facility and cared for by the veterinary staff from the Penn State University Department of Comparative Medicine, Hershey, Pennsylvania. The study protocol was in compliance with the U.S. Department of Agriculture Welfare Act and was approved by the Institutional Animal Care and Use Committee.
Endoscope and instruments
A prototype 12-mm therapeutic double-channel gastroduodenoscope or “R-scope” (XGIF-2TQ160R; Olympus, Tokyo, Japan) was utilized for all procedures. The endoscope and all reusable, non-autoclavable endoscopic equipment (i.e., insulated tip knife, needle knife, and injection needle) were cleansed and chemically sterilized with 2.4% glutaraldehyde (Cidex; Johnson and Johnson, New Brunswick, NJ) and were air-dried before the start of the procedure. All reusable equipment capable of undergoing steam sterilization (i.e., endoscopic forceps and graspers) was autoclaved. Endoclips were supplied by Boston Scientific Corp, Natick, MA (Resolution Clips), and Olympus America Inc, Center Valley, PA (QuickClips).
Biomaterials
The biomaterial used in this study is a product manufactured by LifeCell Corporation (Branchburg, NJ). The matrix is an acellular porcine dermal matrix that was processed in a similar fashion to Strattice™ (LifeCell) [18], in which the matrix undergoes non-damaging proprietary processing that removes all cellular components. However, unlike Strattice™, the APDM in this study did not undergo the α-1,3-galactosyl removal treatment, a key feature believed to minimize the rejection response [19]. The presence of α-1,3-galactosyl does not alter the structural integrity of the acellular matrix, and as this is a porcine model, there is no risk of a rejection response. This biomaterial was also chosen because the reduced processing steps minimize the cost without altering the integrity of the matrix. The biomaterial is supplied as a moist and hydrated matrix in sterile packaging. A piece of matrix approximately 6 cm long × 2 cm wide was trimmed and soaked in room-temperature normal saline solution for at least 4 min prior to its use.
Preoperative care
Animals were fed standard chow ad libitum during their required quarantine period. A liquid diet (Boost; Nestle Nutrition) was given in the 48 h prior to the procedure. The animals were kept without food (access to water ad libitum) beginning 12 h prior to the procedure.
Operative procedure
General anesthesia was induced by an intramuscular injection of telazol (500 μg/kg), medetomidine (70–80 μg/kg), and butorphanol (300 μg/kg). After endotracheal intubation, animals were mechanically ventilated, and anesthesia was maintained with 1–2% isoflurane delivered in 100% O2. End-tidal CO2, SpO2, respiratory rate, respiratory flow-volume curves, temperature, and pulse rate were monitored throughout the procedure. Temperature was maintained by means of a warming blanket and forced warm air sheet. One gram of intravenous cefazolin, 600,000 units of intramuscular penicillin G benzathine, plus penicillin G procaine-based antibiotic were given 30 min before the start of the procedure.
The animal was placed in the supine position, and the oral cavity was cleansed with 10% povidone-iodine solution. The endoscope was then introduced and the stomach contents were aspirated and the stomach was lavaged with 250 ml of 10% povidone-iodine solution. A custom-made intestinal occlusion catheter was introduced into the duodenal bulb to prevent small bowel distension during the procedure. The balloon was inflated with air at approximately 1 cc/kg. Transgastric peritoneal access was established utilizing the previously described STAT technique [14–16]. A mucosal pillow was created on the posterior cardiac portion of the stomach approximately 1 cm distal to the gastroesophageal junction, and 10 ml of normal saline was injected into the submucosal plane with an injection needle (Carr-Locke; US Endoscopy Inc.). A 1–1.5 cm incision was then made over the pillow with a 4-mm needle knife (Huibregst; Wilson-Cooke Medical Inc.). A rat-tooth grasping forceps (FG-47L; Olympus Ltd.) was then used to dissect the loose areolar tissue within the submucosal plane to create a tunnel 6–8 cm long. The tunnel was directed inferiorly along the posterior gastric wall and ended prior to reaching the greater curvature of the stomach. This was previously reported to be the optimal location of the tunnel to target the uterine horns [4]. Prior to creating the seromuscular incision and entering the abdomen, the matrix was loaded onto the endoscope and anchored to the tunnel by one of two techniques: a carpet roll or a drape.
The carpet roll technique was utilized in the first two survival animals. In this technique, sutures were placed to create a loop on the corner or edge of one end of the matrix and another on the opposite end which served as a tail to unroll the matrix. The matrix was then rolled so that the knot on the tail suture was buried in the center. The matrix was grasped with forceps graspers while an endoclip was hooked onto the corner loop suture (Fig. 1A, B). Next, the matrix was introduced with the endoscope into the esophagus and then into the STAT tunnel. It was then anchored by clipping the loop suture to the distal end of the STAT tunnel with the endoclip. At approximately 2 cm proximal to the anchored matrix, a small seromuscular incision was made utilizing a standard needle knife. The incision was then extended to 1.5 cm with an insulated-tip needle knife (IT knife; Olympus Ltd.). After the completion of the intra-abdominal procedure, the tail suture was grasped in order to unroll the matrix over the seromuscular incision (Fig. 2).
APDM preparation and loading. A In the carpet roll technique sutures are placed to create a loop on the corner(s) of one end of the matrix and another loop on the opposite end which serves as a tail to unroll the matrix. B The matrix is then rolled such that the knot on the tail suture is buried in the center. The matrix is grasped with forceps graspers. Prior to anchoring, an endoclip is hooked onto the corner loop suture (not shown). C In the drape technique, the Olympus prototype T-tag device is used to deploy Endo Stitches™ (Covidien, Mansfield, MA), which are secured to the corners of one side of the matrix. Bone wax is used to secure the Endo Stitch™ to the needle catheter of the prototype device
Carpet roll technique. A The matrix is anchored by clipping the loop suture to distal end of the STAT tunnel with endoclips. B A seromuscular incision is made approximately 2 cm proximal to the matrix to access the abdomen. C The tail suture is grasped in order to unroll the matrix over the seromuscular incision. D The tunnel is closed by re-approximating the submucosal and/or mucosal layers with endoclips
In the remaining three survival animals, the drape technique was performed by utilizing the Olympus prototype T-tag device, which consisted of two retractable 18-G needle catheters as previously described [20] with 2-0 Endo Stitch™ (Covidien, Mansfield, MA). After both needle catheters were introduced into the working channels of the R-scope, the catheter was advanced beyond its protective plastic sheath to expose their hollow distal tip, where the needle of the Endo Stitch™ was loaded and secured with the application of bone wax. After the needle catheters were retracted back into their plastic sheaths, the suture ends of the Endo Stitch™ were trimmed to approximately 15–20 cm and tied to two corners of the matrix (Fig. 1C). The matrix was introduced into the esophagus and STAT tunnel parallel to the endoscope and attached to the prototype T-tag device. Once at the end of the tunnel, the needle catheters were punctured into the mucosa of the tunnel in order to deploy the needle of the Endo Stitch™ into the gastric lumen. As described above, the seromuscular layer was incised approximately 1–2 cm proximal to the end of the tunnel to achieve abdominal access. After completion of the intra-abdominal procedure, the needles of the two deployed Endo Stitches™ were grasped by endoscopic forceps and dragged toward the pylorus or the gastroesophageal junction so that the attached matrix slid into the tunnel and occluded the seromuscular incision (Fig. 3).
Drape technique. A Endo Stitches™ are deployed using the Olympus prototype T-tag device on the mucosal surface at the distal end of the STAT tunnel. B A seromuscular incision is made approximately 2 cm proximal to access the abdomen. C Outside the tunnel the deployed Endo Stitches™ are grasped with forceps and dragged toward the pylorus or esophagus such that the attached matrix slides into the tunnel and occludes the seromuscular incision. D The tunnel is closed by re-approximating the submucosal and/or mucosal layers with endoclips
Once intra-abdominal access was achieved, pneumoperitoneum was established with the judicious use of the endoscope air pump. A brief peritoneoscopy was performed to look for evidence of iatrogenic injury. Afterward, a uterine horn resection was performed using previously described techniques [3, 21–23]. Briefly, once the left uterine horn and broad ligaments were identified, a detachable, polymer cinch-loop (Endoloop, Olympus) was placed. A snare cautery was then performed above the Endoloop to resect the specimen. At the conclusion of the procedure the pneumoperitoneum was evacuated by using the suction channel of the endoscope. The matrix was then implanted into the submucosal tunnel by either unrolling or sliding it over the seromuscular incision, as described above. The tunnel was closed by re-approximating the submucosal and/or mucosal layers with endoclips (Resolution, Boston Scientific; QuickClip, Olympus).
Postoperative care
Buprenorphine (0.005–0.01 mg/kg) i.m. was given at the conclusion of the procedure and then every 6–12 h as needed for analgesia. A regular diet was restarted the morning after the procedure. The animals had immediate free access to water and were monitored twice daily for evidence of altered behavior, clinical distress, and pain. Animals were weighed weekly during their 3 week survival period to assess for appropriate weight gain. All animals were euthanized with an overdose of pentobarbital sodium (>100 mg/kg i.v.) after a 3 week survival period.
Necropsy
Immediately after euthanasia a necropsy was performed to evaluate for signs of peritonitis, bleeding, formation of intra-abdominal abscesses and adhesions, and complications within the submucosal tunnel, with particular attention given to the examination of the biomaterial. Following this, tissue specimens were taken from the entire length of the submucosal tract (mucosal incision, seromuscular incision, distal to matrix). Afterward, the specimens were fixed in 10% formalin solution, embedded in paraffin, sectioned on a microtome, and stained with Harris-modified hematoxylin and eosin solution. All slides were then evaluated by a dedicated veterinary histopathologist to assess for microabscess formation and healing of the tunnel.
Data collection
Operative procedures and necropsy were digitally video recorded using Pinnacle Studio Plus 700 USB ver. 10 (Pinnacle Systems, Mountain View, CA) on a Dell Latitude laptop computer (D820; Dell Inc, Round Rock, TX). Significant portions of the procedures and necropsy were also captured on video using a Sony Digital Handycam (DCR-VX2000; Sony Corp, Tokyo, Japan).
Results
The APDM was successfully implanted within the gastric STAT tunnel in all five animals (Fig. 4). Mean operating room (OR) time was 151 ± 68 min. Mean length of the tunnels was 8.2 ± 0.5 cm at the time of the procedure. Mean tunneling time (from submucosal injection to just prior to anchoring the matrix) was 13.8 ± 8.2 min. Mean procedure time was 4 ± 1 min to anchor and 9 ± 12 min to implant the matrix within the tunnel. The uterine horn could not be identified in the second survival animal. Mean uterine horn resection time for the remaining four animals was 12.3 ± 4.7 min. The mean tunnel open time (from seromuscular incision to mucosal clip placement) was 53 ± 18.1 min. The average dimensions of the matrix used were 6.4 ± 0.5 cm long and 1.9 ± 0.4 cm wide. The number of endoclips used to close the mucosal incision ranged from one to three.
It took longer to prepare and load the matrix onto the endoscope with the carpet roll technique because of difficulty rolling and maintaining the matrix as a roll. Twisting of the APDM made it difficult to unroll the matrix. The drape technique, which proved to be faster to load onto the endoscope, was used on the remaining three survival animals. There were minor problems with entanglement of the traction sutures and forceps, and one time the matrix was inadvertently dragged into the peritoneal cavity with the endoscope.
There were no mucosal perforations along the tunnel. A small tear at the mucosal incision, approximately 2 cm long and toward the left, was noted in the second and fourth animals. Otherwise all intraoperative complications were unrelated to the STAT tunnel. In one animal, an inadvertent small bowel perforation occurred, and in a second animal, an endoloop was placed on the small bowel. Both complications required minilaparotomies to be performed. After the fascia was closed, the NOTES procedure continued.
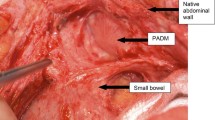
All animals survived for 3 weeks without complications. Mean weight gain at the time of necropsy was 10 ± 2.2 kg. The mucosal and serosal incisions, as well as the remainder of the STAT tunnel, were well healed in all animals and without evidence of necrosis. The matrix was easily identified by its impression on the mucosal surface (Fig. 5).The matrix in the first animal was embedded within the tunnel with one twist on its longitudinal axis. The second animal had partial prolapse of the matrix into the abdomen, with adhesions to the small bowel and spleen. Otherwise, gross inspection was unremarkable for any submucosal or intra-abdominal abscesses, and adhesions to the seromuscular incision were minimal. The matrix demonstrated good adherence to the mucosa and seromuscular layer (Fig. 6).
Histopathology demonstrated a local inflammatory process and fibrovascular ingrowth consistent with robust matrix incorporation into the gastric wall (Figs. 7, 8, 9). One clinically insignificant microabscess was observed within the matrix in the fifth animal.
Discussion
In this porcine survival study, an acellular porcine dermal matrix was investigated as a possible facilitator in NOTES gastrotomy closure. The APDM was quickly and successfully implanted within the gastric submucosal plane in five animals. Despite some suture entanglement early on, the drape technique was found to be more favorable than the carpet roll technique. The latter technique posed difficulty with respect to maintaining the rolled configuration while loading the matrix onto the endoscope and anchoring it within the tunnel. It posed additional problems when attempting to unroll the matrix within the tunnel. The drape technique was deemed easier to load onto the endoscope and implant into the tunnel. The two minor mucosal incision tears at the entrance of the STAT tunnel were inconsequential. One received an extra clip at the time of closure and neither proved to be problematic. In our experience the mucosal incision can sometimes tear toward the left in the porcine stomach when enough pressure is exerted on a looped endoscope. No intraoperative complications related to STAT or matrix implantation were seen. None of the animals demonstrated any gastrotomy leaks or any clinical signs of infection during the survival period. At the time of necropsy, all but the second animal had complete occlusion of the serotomy by the matrix. The second animal had a tongue of matrix that partially prolapsed through the seromuscular incision. Technical error is the most likely explanation and that the anchoring clip during the carpet technique became dislodged at the time of unrolling the matrix.
As expected, histopathology revealed a mild to moderate local inflammatory process at the interface of the matrix and submucosa. There was one microabscess identified within the matrix in the fifth animal. This was ultimately insignificant as the animal showed no clinical signs of infection. In our experience microabscesses have been seen with the STAT tunnel, but to date all have been clinically insignificant [6]. They may represent a normal finding in the healing of this type of gastric incision as microabscess formation has also been observed in non-tunneled transluminal access sites [13, 24]. Remarkably, there is evidence of matrix incorporation into the gastric wall as early as 3 weeks. Plump fibroblast migration into matrix accompanied by endothelial cells confirms that the APDM functions as a scaffold sufficient for fibroblast infiltration and neovascularization, consistent with the findings from other porcine dermal matrices [15, 25] and its human counterpart, Alloderm™ (LifeCell) [10, 13, 26]. Further long-term survival studies are needed to determine whether the APDM also becomes completely remodeled and replaced by the surrounding host tissue.
As evident from the 4th annual NOSCAR meeting, a consensus on intra-abdominal access and closure techniques for NOTES is lacking, with the majority of the attendants awaiting endoscopic suturing devices that overcome obstacles to transgastric access. Our working group has concentrated its research on the development of STAT for peroral transgastric abdominal access. The major benefits of STAT come from the inherent nature of the Z-tract which: (1) anatomically separates the mucosal incision from the serotomy thereby minimizing gastric leaks, (2) allows for some stabilization of the endoscope and after withdrawal of the scope, (3) self-approximates such that layers of the gastric wall seal off the peritoneal access site. This last feature is most prominent with long tunnels and elevated intragastric pressures. Prior work has shown STAT to be a safe, reliable, and reproducible transgastric access technique that provides excellent leak-proof gastrotomy closure. Furthermore, STAT can be accomplished with readily available devices. Additional support for this technique was reported by von Delius et al. [27], whose investigation comparing various gastric closure techniques demonstrated that the extended submucosal tunnel provides the most leak tightness, even when compared to hand-sewn gastrotomy closure. In fact, according to their data, gastric rupture was more likely to occur elsewhere in the stomach than at the submucosal tunnel. In Japan, clinical application of the STAT tunnel has been successfully utilized [28]. In this study we investigated the feasibility of improving the gastrotomy closure of the STAT tunnel with a bioprosthetic mesh implantation without compromising on the benefits of STAT.
Prior investigations, like the submucosal endoscopy with mucosal flap safety valve (SEMF), have demonstrated delayed gastrotomy leak from mucosal necrosis after the creation of large-sized mucosal flaps and a pseudodiverticulum at the myotomy site in a transesophageal tunnel [29, 30]. The utility of APDM or other bioprosthetic material may be most beneficial when there is inadvertent mucosal perforation along the tunnel or a significant seromuscular defect after organ retrieval. Large mucosal perforations within the tunnel will shorten or eliminate the distance between the mucosal and seromuscular incision and may increase the risk for gastrotomy leaks. Intramural implantation of a bioprosthetic material at the site of puncture could help avert gastrotomy leaks and peritonitis. Unanticipated size mismatch between the tunnel and the organ specimen may create a tear along the seromuscular incision that could be large enough to potentially cause mucosal herniation or the formation of a pseudodiverticulum. The use of bioprosthetic material could potentially help avoid such complications and provide more robust closure when larger seromuscular incisions are made. Permanent devices like the cardiac septal occluder have been used for this purpose; however, this method relies on implantation of a permanent device in a bacteria-laden field [31]. The ability to implant dermal matrix within a hollow viscus also has implications for organ reconstruction following endoscopic resections of lesions, whether mucosal or full thickness [32, 33].
This study demonstrated the feasibility of implanting a dermal matrix into the STAT tunnel and provided evidence of its incorporation into the gastric wall at 3 weeks. As mentioned earlier, there were no gastrotomy leaks in our five pigs. Whether this is a function of the matrix is difficult to ascertain as the STAT tunnel alone demonstrates a complete gastrotomy closure. However, matrix implantation into the STAT tunnel does have a theoretical advantage with two-layered closure in the short term and possibly re-established integrity of the native submucosa in the long term. In conclusion, intramural APDM bolstering of STAT closure may represent a technically simple way of providing reliable gastrotomy closure for NOTES.
References
Candage R, Jones K, Luchette FA, Sinacore JM, Vandevender D, Reed RL 2nd (2008) Use of human acellular dermal matrix for hernia repair: friend or foe? Surgery 144:703–709
Armour AD, Fish JS, Woodhouse KA, Semple JL (2006) A comparison of human and porcine acellularized dermis: interactions with human fibroblasts in vitro. Plast Reconstr Surg 117(3):845–856
Kim H, Bruen K, Vargo D (2006) Acellular dermal matrix in the management of high-risk abdominal wall defects. Am J Surg 192:705–709
Diaz JJ Jr, Conquest AM, Ferzoco SJ, Vargo D, Miller P, Wu YC, Donahue R (2009) Multi-institutional experience using human acellular dermal matrix for ventral hernia repair in a compromised surgical field. Arch Surg 144(3):209–215
Wainwright DJ (1995) Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns 21(4):243–248
Breuing KH, Warren SM (2005) Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg 55(3):232–239
Chaplin JM, Costantino PD, Wolpoe ME, Bederson JB, Griffey ES, Zhang WX (1999) Use of an acellular dermal allograft for dural replacement: an experimental study. Neurosurgery 45(2):320–327
Akbal C, Lee SD, Packer SC, Davis MM, Rink RC, Kaefer M (2006) Bladder augmentation with acellular dermal biomatrix in a diseased animal model. J Urol 176(4 Pt 2):1706–1711
Beniker D, McQuillan D, Livesey S, Urban RM, Turner TM, Blum B, Hughes K, Haggard WO (2003) The use of acellular dermal matrix as a scaffold for periosteum replacement. Orthopedics 26(5 Suppl):s591–s596
Rattner D, Kalloo A, ASGE/SAGES Working Group (2006) ASGE/SAGES Working group on natural orifice translumenal endoscopic surgery. Surg Endosc 20(2):329–333
McGee MF, Marks JM, Onders RP, Chak A, Rosen MJ, Williams CP, Jin J, Schomisch SJ, Ponsky JL, Case Advanced Surgical Endoscopy Team [CASE-T] (2008) Infectious implications in the porcine model of natural orifice transluminal endoscopic surgery (NOTES) with PEG-tube closure: a quantitative bacteriologic study. Gastrointest Endosc 68(2):310–314
Merrifield BF, Wagh MS, Thompson CC (2006) Peroral transgastric organ resection: a feasibility study in pigs. Gastrointest Endosc 63:693–697
Pai RD, Fong DG, Bundga ME, Odze RD, Rattner DW, Thomspon CC (2006) Transcolonic endoscopic cholecystectomy: a NOTES survival study in a porcine model (with video). Gastrointest Endosc 64:428–434
Moyer MT, Pauli EM, Haluck RS, Mathew A (2007) The self-approximating translumenal access technique (STAT) for potential use in NOTES: an ex vivo porcine model (with video). Gastrointest Endosc 66(5):974–978
Pauli EM, Moyer MT, Haluck RS, Mathew A (2008) Self-approximating translumenal access technique (STAT) for NOTES: a porcine survival study (with video). Gastrointest Endosc 67(4):690–697
Pauli EM, Moyer MT, Haluck RS, Mathew A (2010) Directed submucosal tunneling permits in-line endoscopic position for transgastric NOTES (with video). Surg Endosc 24(6):1474–1481
Mathew A, Tomasko JM, Pauli EM, Moyer MT, Gopal J, Ancrile BB, Rogers AM, Haluck RS (2011) Reliability of gastric access closure with the self-approximating transluminal access technique (STAT) for NOTES. Surg Endosc 25(8):2718–2724
Connor J, McQuillan D, Sandor M, Wan H, Lombardi J, Bachrach N, Harper J, Xu H (2009) Retention of structural and biochemical integrity in a biological mesh supports tissue remodeling in a primate abdominal wall model. Regen Med 4(2):185–195
Xu H, Hua W, Zuo W, Sun W, Owens RT, Harper JR, Avares DL, McQuillan DJ (2009) A porcine-derived acellular dermal scaffold that supports soft tissue regeneration: removal of terminal galactose-alpha-(1,3)-galactose and retention of matrix structure. Tissue Eng 15(7):1807–1819
Sumiyama K, Gostout CJ, Rajan E, Bakken TA, Deters JL, Knipschield MA (2007) Endoscopic full-thickness closure of large gastric perforations by use of tissue anchors. Gastrointest Endosc 65:134–139
Sumiyama K, Gostout CJ, Rajan E, Bakken TA, Deters JL, Knipschield MA, Hawes RH, Kalloo AN, Pasricha PJ, Chung S, Kantsevoy SV, Cotton PB (2006) Pilot study of the porcine uterine horn as an in vivo appendicitis model for development of endoscopic transgastric appendectomy. Gastrointest Endosc 64(5):808–812
Wagh MS, Merrifield BF, Thompson CC (2005) Endoscopic transgastric abdominal exploration and organ resection: initial experience in a porcine model. Clin Gastroenterol Hepatol 3(9):892–896
Wagh MS, Merrifield BF, Thoompson CC (2006) Survival studies after endoscopic transgastric oophorectomy and tubectomy in a porcine model. Gastrointest Endosc 63(3):473–478
Kalloo AN, Singh VK, Jagannath SB, Niiyama H, Hill SL, Vaughn CA, Magee CA, Kantsevoy SV (2004) Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions. Gastrointest Endosc 60:114–117
Silverman RP, Li EN, Holton LH 3rd, Sawan KT, Goldberg NH (2004) Ventral hernia repair using allogenic acellular dermal matrix in a swine model. Hernia 8(4):336–342
Kirschner RE, Cabiling DS, Slemp AE, Siddiqi F, LaRossa DD, Losee JE (2006) Repair of oronasal fistulae with acellular dermal matrices. Plast Reconstr Surg 118(6):1431–1440
von Delius S, Gillen S, Doundoulakis E, Schneider A, Wilhelm D, Fiolka A, Wagenpfeil S, Schmid RM, Feussner H, Meining A (2008) Comparison of transgastric access techniques for natural orifice transluminal endoscopic surgery. Gastrointest Endosc 68(5):940–946
Inoue H, Minami H, Kobayashi Y, Sato Y, Kaga M, Suzuki M, Satodate H, Odaka N, Itoh H, Kudo S (2010) Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy 42(4):265–271
Sumiyama K, Gostout CJ, Rajan E, Bakken TA, Knipschield MA, Chung S, Cotton PB, Hawes RH, Kalloo AN, Kantsevoy SV, Pasricha PJ (2007) Transgastric cholecystectomy: transgastric accessibility to the gallbladder improved with the SEMF method and a novel multibending therapeutic endoscope. Gastrointest Endosc 65(7):1028–1034
Sumiyama K, Gostout CJ, Rajan E, Bakken TA, Knipschield MA, Chung S, Cotton PB, Hawes RH, Kalloo AN, Kantsevoy SV, Pasricha PJ (2008) Pilot study of transesophageal endoscopic epicardial coagulation by submucosal endoscopy with the mucosal flap safety valve technique (with videos). Gastrointest Endosc 67(3):497–501
Perretta S, Sereno S, Forgione A, Dallemagne B, Coumaros D, Boosfeld C, Moll C, Marescaux J (2007) A new method to close the gastrotomy by using a cardiac septal occluder: long-term survival study in a porcine model. Gastrointest Endosc 66(4):809–813
Nieponice A, McGrath L, Qureshi I, Beckman EJ, Luketich JD, Gilbert TW, Badylak SF (2009) An extracellular matrix scaffold for esophageal stricture prevention after circumferential EMR. Gastrointest Endosc 69(2):289–296
Reider E, Martinec DV, Dunst CM, Swanstrom LL (2011) A novel technique for natural orifice endoscopic full-thickness colon wall resection: an experimental pilot study. J Am Coll Surg 213(3):422–429
Acknowledgments
Bioprosthetic mesh and funding for this study were generously provided by LifeCell Corporation (Branchburg, NJ). We thank the staff at the Department of Comparative Medicine for their care of the animals (Dr. Ronald Wilson, Dr. Elizabeth Carney, and Mrs. Joy Ellwanger) and for the histopathology analysis (Dr. Timothy K. Cooper). We also acknowledge Olympus (R-scope and accessories) and Boston Scientific (accessories).
Disclosures
Drs. Jegan Gopal, Eric M. Pauli, Randy S. Haluck, Matthew T. Moyer, and Abraham Mathew have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gopal, J., Pauli, E.M., Haluck, R.S. et al. Intramural acellular porcine dermal matrix (APDM)-assisted gastrotomy closure for natural orifice transluminal endoscopic surgery (NOTES). Surg Endosc 26, 2322–2330 (2012). https://doi.org/10.1007/s00464-012-2183-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-012-2183-x