Abstract
Background
Various antiadhesive coatings have been proposed for intraperitoneal onlay meshes (IPOM). However, adhesions, mesh infections, and impaired integration remain clinically relevant problems. In this experiment, human vital amniotic membrane (AM) was tested as antiadhesive mesh coating. Vital AM complies with clinical standards of product safety.
Methods
In this study, 24 rats were randomized to one control or two treatment groups (n = 8). An uncoated polypropylene mesh (Vitamesh) was implanted using open IPOM technique and fixed with four sutures. In the treatment groups, vital AM was attached to Vitamesh by fibrin sealant fixation. The observation period was 7 and 17 days. Vitamesh fixed by suture only served as the control condition (17 days). Adhesion formation, tissue integration, and neovascularization were assessed macroscopically and histologically.
Results
All the meshes in the control group elicited severe adhesions. Vital AM was highly efficient in reducing adhesions to mesh and sutures. No foreign body reaction or unfavorable immunologic response to vital AM occurred. Tissue integration and neovascularization of coated meshes were good. Fibrin sealant yielded a reliable fixation.
Conclusion
Human vital AM was highly effective in reducing adhesions to polypropylene mesh and sutures in experimental IPOM. No adverse effects were detected, and tissue integration of the mesh was good.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Intraabdominal peritoneal onlay mesh repair (IPOM) can be considered a well-established technique for selected patients [1, 2]. The laparoscopic view allows detection of multiple and occult hernia defects and offers undeniable advantages, especially for obese patients [3, 4].
For more than two decades, extended polytetrafluoroethylene (ePTFE) was the material of choice for antiadhesive coating of IPOM meshes [4, 5]. However, its undisputed efficacy for adhesion prevention is opposed by its susceptibility to infection [6]. The incidence of ePTFE mesh infection is as high as 10% in the literature and usually requires explantation [7, 8]. Shrinkage, wrinkling, and seroma formation are other frequent complications of ePTFE, leading to the development of new mesh coatings [9, 10].
Polylactic acid, methyl cellulose, and collagen matrices, currently used for adhesion prevention in modern IPOM meshes, are less susceptible to infection [11, 12]. However, adhesions remain a clinically relevant problem, which is not restricted to mesh technology. It becomes apparent that the formation of adhesions to perforating fixation devices (PFDs) is a frequent cause for small bowel obstruction [13–15].
Consequently, an optimized interaction of mesh and PFD seems desirable. This formed the rationale for testing human vital amniotic membrane (AM) in an experimental model of IPOM and fixing it to the mesh with fibrin sealant [16, 17]. This biologic matrix has been used successfully for wound repair and regeneration [18–20].
A vital AM was used with mixed results in experimental hernia research almost a decade ago [16]. However, the irradiation and chemical processing required for devitalization of AM seemed a major disadvantage to us due to the inalterable deletion of adherent multi- and pluripotent stem cells [21–23]. Pluripotent stem cell marker-positive cells are scattered in the amniotic epithelium [24].
The primary outcome parameters in our study were adhesion formation and foreign body reaction. The secondary outcome parameters were tissue integration and dislocation of vital AM attached to the mesh by means of fibrin sealant only.
Methods
Male Sprague-Dawley rats weighing 400–450 g were obtained from the Institut fuer Labortierkunde und genetik der Medizinischen Fakultaet der Universitaet Wien, Himberg, Austria. The mesh used in this study was Vitamesh, a lightweight macroporpous polypropylene mesh manufactured by Proxy Medical (Galway, Ireland). The fibrin sealant was Artiss (four units of thrombin) manufactured by Baxter (Vienna, Austria).
The mesh and fibrin sealant were supplied by courtesy of their manufacturers. Vital AM was obtained from the Red Cross Blood Transfusion Service of Upper Austria, produced under “good manufacturing practice” (GMP) conditions. All reagents used were of analytical grade, and surgery was performed under sterile conditions at the Ludwig Boltzmann Institute in Vienna. The study protocol was approved by the authority of the Vienna city government.
Group randomization
The 23 rats in this study were randomized to one control and two treatment groups as follows:
-
Control group (n = 8): Vitamesh was fixed by suture only, and the observation period was 17 days. Although an uncoated polypropylene mesh should not be used clinically in IPOM repair, it was chosen as a reliable and reproducible control group substance.
-
Treatment group 1 (T1; n = 8): Vitamesh and PFD were covered with vital AM, the observation period was 7 days.
-
Treatment group 2 (T2; n = 8): Vitamesh and PFD were covered with vital AM, and the observation period was 17 days.
The observation periods were chosen to detect early adhesion formation, dislocation of vital AM after full degradation of fibrin sealant, and possible foreign body reaction to the xenograft.
Processing of vital amnion
Placentas after cesarean section were collected with informed consent from the donors. Further processing was performed as described previously [17]. Briefly, AM was peeled off the placenta and washed extensively with phosphate-buffered saline (PBS). Subsequently, 3 × 3-cm AM transplants for grafting as well as microbiologic and viability testing were prepared. For microbiologic and initial viability testing, punch biopsies (diameter, 8 mm) were taken from a separate 3 × 3-cm transplant.
The AM grafts were attached to nitrocellulose disks (Millipore, Billerica, USA) and wrapped in 15-ml tubes with 10 ml of a cryoprotective medium containing RPMI 1640 (PAA, Pasching, Austria), FCS (PAA), DMSO (WAK Chemie, Steinbach, Germany), l-Glut (PAA), and an antibiotic–antimycotic solution (PAA). The transplants then were frozen with a controlled-rate freezer and cryopreserved at −80°C.
Before in vivo application, the AM grafts were thawed and washed several times with PBS. One cryopreserved transplant was taken for residual viability testing with the EZ4U cell proliferation and cytotoxicity assay (Biomedica, Vienna, Austria) as described by Hennerbichler et al. [17] (Fig. 1). All AM transplants were microbiologically negative and viable.
Implant size
The Vitamesh was precut to 2 × 2 cm2 in the rats. The size of the implants was fitted to the anatomic and technical requirements (i.e., adequate distance to wound margins and explantation without mechanical damage to the samples). Vital AM was 3 × 3 cm2 in T1 and T2.
Surgical model
The described procedure reproduces the open IPOM technique, placing the implant on the intact peritoneum and attaching it to the abdominal wall with a PFD [13].
Surgery in rats
The 23 rats were anesthetized with an intramuscular injection of Ketavet (ketamine-hydrochloride 100 mg/ml; Pharmacia, Erlangen, Germany) and Rompun (xylazine-hydrochloride; Bayer, Leverkusen, Germany).
The abdomen was thoroughly shaved, and skin disinfection was performed. Subsequently, the skin was incised with a scalpel, and the subcutaneous fat tissue was bluntly detached from the abdominal muscles. A U-shaped laparotomy was made at the epigastral level from left to right, beginning and ending about 1.5 cm under the lateral rib cage. The abdominal wall was flipped caudally and the peritoneum exposed, allowing a direct view of the implant site. The mesh was placed on the peritoneum in a midline position with a distance of at least 1 cm between the implant margins and the incision. The mesh was sutured at all four corners of the Vitamesh (Synthofil 4/0; Ethicon, Norderstedt, Germany).
In the control group, the operation ended at this point. In the treatment groups (T1, T2), vital AM was attached to the Vitamesh with 0.2 ml of fibrin sealant. The mesh was spray-covered with the fibrin sealant (Easy Spray; Baxter, Vienna, Austria). The skin incision was closed in anatomic layers, and 1 ml of physiologic saline was administered subcutaneously to compensate for dehydration.
Postoperative care of the rats
The rats were kept in single cages during the remaining observation periods and checked daily for signs of infection, seroma formation, or abscess formation. Analgesic treatment (2 mg/kg bodyweight intramuscular application of Temgesic [buprenorphine], Merck, Vienna, Austria) was routinely applied once daily for 3 days postoperatively.
Autopsy of the rats
The rats were killed as scheduled in the randomization protocol under anesthesia by an intravenous injection of thiopental 1 ml (1 g; Sandoz, Kundl, Austria).
Macroscopy
Seroma formation, signs of local inflammation, and tissue integration were independently assessed by two investigators blinded to group assignment. The macroscopic score, described previously, is based on an A (no alteration) B (modest alteration) C (severe alteration) scale that already has been used reliably in studies on bio- and synthetic meshes [13].
Seroma formation
Absence of seroma was scored as A. A seroma (encapsulation with fluid) closely adjacent to the implant or containing less than 0.5 ml of fluid (verified by needle aspiration) was scored as B, and a seroma formation containing more than 0.5 ml of fluid was scored as C.
Local inflammation
No visible inflammation (defined as unfavorable inflammation with pus and debris) was scored as A. Small amounts of debris and pus were scored as B, and abscess formation was scored as C.
Tissue integration
Complete integration of the whole implant (tissue ingrowth and vascularization visible to the naked eye) was scored as A. An implant only partly integrated (<50% of surface area) was scored as B, whereas no detectable integration (e.g., no tissue ingrowth through the interstices of the mesh, edges of the implant not integrated) was scored as C.
Dislocation
Dislocation was defined as visible detachment of the mesh from the underlying abdominal wall. The implant found in its original position with all four edges adjacent to abdominal wall was scored as A. An implant with up to 30% of its surface area detached was scored as B, and any dislocation more severe (>30% free floating in the abdominal cavity) was scored as C. Failure of a suture knot led to a scoring of A in favor of the implant material. The dislocation of AM from Vitamesh was scored accordingly. A shrinkage of the mesh less than 10% of its original size was scored as A. Shrinkage of 10–30% was scored as B, and more than 30% shrinkage was scored as C.
Adhesions
Adhesions in the rats were scored according to the score first described by Vandendael (Table 1). This score offers the advantage of combining precise parameters describing strength, width, and amount of adhesion.
Histology
After macroscopic evaluation, all samples were fixed in 10% buffered formaldehyde solution (Merck) and embedded in paraffin. For the distinction of native and human (AM) collagen, 5-μm sections were stained with hematoxylin and eosin (H&E) and Picrosirius red. Blinded analysis and grading for the following parameters were performed [25]:
-
Foreign body reaction (defined as prolonged neutrophil response, foreign body giant cells, and necrosis)
-
Macrophages
-
Lymphocytes and plasma cells
-
Tissue integration based on neovascularization and fibroblast ingrowth.
Histologic grading
The histologic grading scale consisted of 0 (no alteration), 1 (moderate alteration), 2 (strong alteration), and 3 (maximum alteration) compared with the tissue of native rats. This histologic grading system has been described previously [25].
Statistical analysis
Statistical analysis of the Vandendael adhesion scores between the treatment and the control groups was performed. The Kruskal-Wallis test and the Mann-Whitney U test were applied. A P value less than 0.005 was considered statistically significant.
Results
Intraoperative observations
The handling of vital AM was superb due to the clear distinction between the visceral and parietal sides. Vital AM easily slipped on mesh surface. The slow-clotting fibrin sealant allowed repositioning for 120 s.
Macroscopy
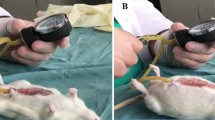
Excellent results were achieved in the treatment groups (T1 and T2) for the parameters “seroma formation,” “local inflammation,” “tissue integration,” “dislocation,” and “shrinkage,” which, based on their absence, could all be scored A (Fig. 2). No macroscopic signs of unfavorable inflammation occurred in any sample, but neovascularization and an equal integration of the mesh were detectable. The meshes were firmly in place at autopsy, and no dislocation was found. The presence of vital AM amnion could not be verified macroscopically. Vitamesh showed no signs of shrinkage or contraction by surrounding scar tissue.
17 days after implantation VITAL AM coated meshes appeared well integrated and were usually spared from adhesion formation. This Figure illustrates that even the sutures which have been covered by VITAL AM are free of adhesions. The results obtained with VITAL AM were outstanding in terms of mesh integration and adhesion prevention
In the control group, the meshes were well integrated and free of seromas, but the macroscopic picture was expectedly dominated by massive adhesions and a pronounced local inflammation (score of C).
Adhesion formation
The results are illustrated in Table 1. Vital AM substantially reduced adhesions in T1 and T2. It was most remarkable how mesh and sutures were covered by a homogeneous, transparent cellular layer, interpreted as the ingrown residual vital AM. In T1 (observation period, 7 days) seven of eight meshes showed only mild adhesions (as measured by the Vandendael score) and translated to a clinically unproblematic finding (i.e., low risk of small bowel obstruction). One mesh in T1 showed moderate adhesions.
In T2 (observation period, 17 days), four meshes were free of adhesions, two meshes showed mild adhesions, and two meshes showed moderate adhesions. No mesh in T1 or T2 elicited severe adhesions, and the PFDs also were free of adhesions (Fig. 2).
In the control group, all the meshes were covered with severe adhesions, and it was apparent that the margins of the Vitamesh and sutures were the most critical areas.
The differences between the T1, T2, and control groups were highly significant according to both the Kruskal-Wallis and the Mann-Whitney U tests (P < 0.001). The differences between T1 and T2 were not significant (P = 0.328).
Histology
The H&E staining confirmed the absence of a foreign body reaction to vital AM. The scar formation around AM-coated meshes appeared physiologic, characterized by an equal deposition of collagen and ingrowth of vessels and leading to excellent implant integration. Macrophages, lymphocytes, and plasma cells were sparse, and no signs of an active inflammatory response were detected. These parameters were rated 1 and translate to an excellent performance of the xenogenic vital AM in rats.
Picrosirius red staining [26] was a useful tool for demonstrating the persistence of vital AM, which was already well integrated, providing protection from adhesions (Figs. 3, 4) at both time points of observation. No granulomatous cells were found in its vicinity. The clear distinction of vital AM verified the satisfying biocompatibility. The integration of uncoated meshes was comparable, but foreign body reaction was pronounced (histologic score, 2) due to the direct contact of the polypropylene with the viscerum.
The histology shows that the polypropylene mesh is already incorporated (after 17 days) in the surrounding tissue and that the VITAL AM forms a tightly attached barrier preventing mesh and sutures from adhesions. The foreign body reaction (indicated by the granulamatous cells) is mild and limited to the close environment of the mesh fibers. The staining is Picosirius red which was found very suitable to discern the VITAL AM from the physiological scar tissue elicited by the mesh. The upper image is the view by back-light microscopy, the lower image is polarised
Discussion
Vital AM provided excellent adhesion prevention and showed good biocompatibility, eliciting only a mild local inflammatory response (Fig. 2). The fibrin sealant served as safe fixation of vital AM and prevented its dislocation in the tested observation periods. Our findings with vital AM support the assumption that biologically active coatings could be specifically useful for adhesion prevention and tissue integration in hernia repair.
Voskerician et al. [27, 28] reported similar beneficial results achieved with human peritoneal membrane in experimental IPOM. Viability of AM seemed mandatory for its full benefits and potential to be realized. Vital AM contains pluripotent epithelial and mesenchymal stem cells, which otherwise would have been erased by irradiation or chemical processing [21–23, 29, 30].
As shown by our results, vital AM formed a functional layer that did not impair mesh integration but provided excellent adhesion prevention (Figs. 3, 4). Vital AM showed excellent handling characteristics, which definitely set it apart from many commercially available coatings that are difficult to insert through laparoscopic trocars and sometimes can be altered easily by perforating fixation devices. As an alternative, slow-clotting fibrin sealant allowed manipulation of the vital AM for about 120 s and securely fixed vital AM [31].
Although vital AM was used as a xenograft in our animal model, no foreign body reaction or adverse effects associated with immunogenicity were observed. We are aware of this study’s obvious limitations (e.g., the use of “pure” polypropylene in the control group). The control group was included because IPOM has no commonly accepted standard of care. Furthermore, the implant of uncoated Vitamesh guaranteed reproducible adhesion formation.
Concerning the translational issue, it must be emphasized that vital AM is manufactured by the blood bank of the Red Cross according to clinical GMP standards (Fig. 1) and already is available for clinical applications (i.e., wound dressing) [29, 32].
Conclusions
Vital human amniotic membrane provided excellent antiadhesion in experimental IPOM. Besides its superior handling characteristics, it showed good biocompatibility and rapid integration. As a consequence of our positive findings, we intend to continue the preclinical research in an IPOM trial using pigs and longer observation periods.
References
Benhidjeb T, Benecke C, Strik MW (2008) Incisional hernia repair: sublay or intraperitoneal onlay mesh (IPOM)? Zentralbl Chir 133:458–463
Schug-Pass C, Sommerer F, Tannapfel A, Lippert H, Kockerling F (2009) The use of composite meshes in laparoscopic repair of abdominal wall hernias: are there differences in biocompatibily? Experimental results obtained in a laparoscopic porcine model. Surg Endosc 23:487–495
Ching SS, Sarela AI, Dexter SP, Hayden JD, McMahon MJ (2008) Comparison of early outcomes for laparoscopic ventral hernia repair between nonobese and morbidly obese patient populations. Surg Endosc 22:2244–2250
Palanivelu C, Rangarajan M, Jategaonkar PA, Amar V, Gokul KS, Srikanth B (2009) Laparoscopic repair of diastasis recti using the “Venetian blinds” technique of plication with prosthetic reinforcement: a retrospective study. Hernia 13:287–292
Gananadha S, Samra JS, Smith GS, Smith RC, Leibman S, Hugh TJ (2008) Laparoscopic ePTFE mesh repair of incisional and ventral hernias. ANZ J Surg 78:907–913
Rauth TP, Poulose BK, Nanney LB, Holzman MD (2007) A comparative analysis of expanded polytetrafluoroethylene and small intestinal submucosa: implications for patch repair in ventral herniorrhaphy. J Surg Res 143:43–49
Trunzo JA, Ponsky JL, Jin J, Williams CP, Rosen MJ (2009) A novel approach for salvaging infected prosthetic mesh after ventral hernia repair. Hernia (in press)<AQ11>
Paton BL, Novitsky YW, Zerey M, Sing RF, Kercher KW, Heniford BT (2007) Management of infections of polytetrafluoroethylene-based mesh. Surg Infect Larchmt 8:337–341
Di Mugno M, Runfola M, Magalini S, Sermoneta D, Gui D (2006) Rippled mesh: a CT sign of abdominal wall ePTFE prosthesis infection. G Chir 27:384–387
Tsimoyiannis EC, Tsimogiannis KE, Pappas-Gogos G, Nikas K, Karfis E, Sioziou H (2008) Seroma and recurrence in laparoscopic ventral hernioplasty. JSLS 12:51–57
Burger JW, Halm JA, Wijsmuller AR, ten Raa S, Jeekel J (2006) Evaluation of new prosthetic meshes for ventral hernia repair. Surg Endosc 20:1320–1325
Olmi S, Scaini A, Erba L, Bertolini A, Croce E (2007) Laparoscopic repair of inguinal hernias using an intraperitoneal onlay mesh technique and a Parietex composite mesh fixed with fibrin glue (Tissucol): personal technique and preliminary results. Surg Endosc 21:1961–1964
Petter-Puchner AH, Walder N, Redl H, Schwab R, Ohlinger W, Gruber-Blum S et al (2008) Fibrin sealant (Tissucol) enhances tissue integration of condensed polytetrafluoroethylene meshes and reduces early adhesion formation in experimental intraabdominal peritoneal onlay mesh repair. J Surg Res 150:190–195
Withers L, Rogers A (2006) A spiral tack as a lead point for volvulus. JSLS 10:247–249
Karahasanoglu T, Onur E, Baca B, Hamzaoglu I, Pekmezci S, Boler DE et al (2004) Spiral tacks may contribute to intraabdominal adhesion formation. Surg Today 34:860–864
Szabo A, Haj M, Waxsman I, Eitan A (2000) Evaluation of seprafilm and amniotic membrane as adhesion prophylaxis in mesh repair of abdominal wall hernia in rats. Eur Surg Res 32:125–128
Hennerbichler S, Reichl B, Pleiner D, Gabriel C, Eibl J, Redl H (2007) The influence of various storage conditions on cell viability in amniotic membrane. Cell Tissue Bank 8:1–8
Said DG, Nubile M, Alomar T, Hopkinson A, Gray T, Lowe J et al (2009) Histologic features of transplanted amniotic membrane: implications for corneal wound healing. Ophthalmology 116:1287–1295
Ilancheran S, Moodley Y, Manuelpillai U (2009) Human fetal membranes: a source of stem cells for tissue regeneration and repair? Placenta 30:2–10
Kesting MR, Wolff KD, Hohlweg-Majert B, Steinstraesser L (2008) The role of allogenic amniotic membrane in burn treatment. J Burn Care Res 29:907–916
Toda A, Okabe M, Yoshida T, Nikaido T (2007) The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J Pharmacol Sci 105:215–228
Yu SJ, Soncini M, Kaneko Y, Hess DC, Parolini O, Borlongan CV (2009) Amnion: a potent graft source for cell therapy in stroke. Cell Transplant 18:111–118
Alviano F, Fossati V, Marchionni C, Arpinati M, Bonsi L, Franchina M et al (2007) Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev Biol 7:11
Miki T, Mitamura K, Ross MA, Stolz DB, Strom SC (2007) Identification of stem cell marker-positive cells by immunofluorescence in term human amnion. J Reprod Immunol 75:91–96
Petter-Puchner AH, Fortelny RH, Mittermayr R, Walder N, Ohlinger W, Redl H (2006) Adverse effects of porcine small intestine submucosa implants in experimental ventral hernia repair. Surg Endosc 20:942–946
Mateijsen MA, van der Wal AC, Hendriks PM, Zweers MM, Mulder J, Struijk DG et al (1999) Vascular and interstitial changes in the peritoneum of CAPD patients with peritoneal sclerosis. Perit Dial Int 19:517–525
Voskerician G, Jin J, Hunter SA, Williams CP, White M, Rosen MJ (2009) Human peritoneal membrane reduces the formation of intraabdominal adhesions in ventral hernia repair: experimental study in a chronic hernia rat model. J Surg Res 157:108–114
Jin J, Voskerician G, Hunter SA, McGee MF, Cavazzola LT, Schomisch S et al (2009) Human peritoneal membrane controls adhesion formation and host tissue response following intraabdominal placement in a porcine model. J Surg Res 156:297–304
Parolini O, Alviano F, Bagnara GP, Bilic G, Buhring HJ, Evangelista M et al (2008) Concise review: isolation and characterization of cells from human term placenta: outcome of the first international workshop on placenta derived stem cells. Stem Cells 26:300–311
Wolbank S, Hildner F, Redl H, Van GM, Gabriel C, Hennerbichler S (2009) Impact of human amniotic membrane preparation on release of angiogenic factors. J Tissue Eng Regen Med 3:651–654
Jain AK, Bansal R, Sukhija J (2008) Human amniotic membrane transplantation with fibrin glue in management of primary pterygia: a new tuck-in technique. Cornea 27:94–99
Stadler G, Hennerbichler S, Lindenmair A, Peterbauer A, Hofer K, Van GM et al (2008) Phenotypic shift of human amniotic epithelial cells in culture is associated with reduced osteogenic differentiation in vitro. Cytotherapy 10:743–752
Acknowledgments
This work was partially supported by a Novosanguis grant. We acknowledge the manufacturers’ support with mesh and fibrin sealant and thank Thomas Benesch, PhD, Nadja Walder, MD, Claudia Keibl, Vet, and Alexandra Meinl for their support.
Disclosures
A. H. Petter-Puchner, R. H Fortelny, K. Mika, S. Hennerbichler, H. Redl, and C. Gabriel have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Appendix
Rights and permissions
About this article
Cite this article
Petter-Puchner, A.H., Fortelny, R.H., Mika, K. et al. Human vital amniotic membrane reduces adhesions in experimental intraperitoneal onlay mesh repair. Surg Endosc 25, 2125–2131 (2011). https://doi.org/10.1007/s00464-010-1507-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-010-1507-y