Abstract
Background
Local excision of rectal cancer as an alternative to radical resection for patients with small nonadvanced low rectal cancer (SNALRC) (iT1–iT2, iN0) is debated. This study aimed to analyze the short- and long-term results for a series of 135 patients with SNALRC who underwent local excision by transanal endoscopic microsurgery (TEM).
Methods
According to the study protocol, 135 patients classified by endorectal ultrasound, magnetic resonance imaging (MRI), and computed tomography (CT) imaging as having iT1 iN0 iM0 (n = 51) or iT2 iN0 iM0 (n = 84) low rectal cancer were enrolled in the study. All the patients with iT2 rectal cancer underwent neoadjuvant therapy. The definitive histologic findings showed 24 pT0 patients (17.8%), 66 pT1 patients (48.8%), and 45 pT2 patients (33.4%).
Results
Minor complications were observed in 12 patients (8.8%) and major complications in 2 patients (1.5%). During a median follow-up period of 97 months (range, 55–139 months), local recurrences occurred for four patients and distant metastases for two patients. The patients who experienced a recurrence had been preoperatively staged as iT2 and were low or nonresponders to neoadjuvant treatment (ypT2). At the end of the follow-up period, the disease-free survival rates were 100% for the iT1 patients and 93% for the iT2 patients
Conclusions
The long-term results for adequate local excision by TEM with or without neoadjuvant radiochemotherapy in the treatment of SNALRC based on the current study protocol are not inferior to those reported in the literature for radical surgery with total mesorectal excision (TME).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Colorectal cancer is a common malignant disease with high morbidity and mortality rates. It affects 130,000 American men and women and accounts for 56,000 deaths annually [1]. In the past two decades, several relevant improvements occurred in the diagnosis and treatment of rectal cancer [2]. Routine use of endorectal ultrasound (EUS) combined with new technology (magnetic resonance imaging and computed tomography [CT] scanning) has significantly improved our ability to perform a correct preoperative staging, which is mandatory for the selection of patients eligible to undergo local excision. The introduction of transanal endoscopic microsurgery (TEM) by Gerhard Buess for local excision of benign rectal tumors has been an important achievement.
Currently, most authors consider local excision curative for patients with a primary tumor limited to the mucosa and submucosa (T1N0M0) without cytologic or histologic high-risk features (G1–G2) [3–5]. Once the tumor invades the muscolaris propria (T2), total mesorectal excision (TME) is considered mandatory for all stages of rectal cancer, and low anterior resection (LAR) or, less frequently, abdominal perineal resection (APR) is performed according to the distance of the tumor from the dentate line [6–11]. Both operations are associated with relevant morbidity and mortality rates, particularly among high-risk patients, and they strongly modify the quality of life due to stoma creation and genitourinary sequelae. Impairment of anal sphincter function also is frequently reported after low anastomosis.
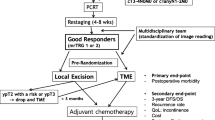
Since 1992, to reduce morbidity and mortality rates, functional sequelae, and stoma creation rates, a protocol that includes preoperative high-dose radiotherapy, associated with 5-fluorouracil (5-FU) since 1997, followed by local excision with TEM in selected cases has been used. In this report, we define as small nonadvanced low rectal cancer (SNALRC) all iT1 N0 and iT2 N0 lesions with a diameter of 3 cm or less.
This article aims to report the results for patients with SNALRC who underwent local excision according to an original surgical technique performed by TEM and to evaluate the short- and long-term outcomes of this treatment for iT1 and iT2, iN0 patients.
Materials and methods
Patient selection
From 1992 to 2005, a cohort of 516 patients undergoing TEM were enrolled in a prospective study. Of these patients, 320 patients had rectal adenoma, and 196 had rectal cancer (51 patients with iT1 iN0 iM0, 84 patients with iT2 iN0 iM0, and 61 patients with iT3 iN0 iM0 SNALRC). For 135 patients (26.2%; 92 men and 43 women; 51 iT1 iN0 iM0 and 84 iT2 iN0 iM0 patients), TEM was performed with curative intent. The median age of these patients was 62 years (range, 55–70 years), and the mean age was 63 years (range, 31–88 years). The patients with iT1 tumor were excluded if the lesion was even partially located in the intraperitoneal rectum.
The inclusion criteria for patients with iT1 and iT2 rectal cancer specified tumor located within 8 cm from the anal verge and a tumor diameter of 3 cm or less. Patients were enrolled independently of cancer grading. Only mucinous and highly undifferentiated invasive tumors were excluded. All patients included in the current study had tumors not fixed at palpation.
All patients were informed concerning the oncologic risk (local recurrence and distant metastases) of local excision and its possible complications (e.g., bleeding, suture dehiscence, temporary gas or stool incontinence, conversion to laparotomy with colonic resection and colostomy) and signed a detailed consent form. All patients consented to be enrolled in a close follow-up program.
For each patient, history and routine laboratory tests, including tumor markers’ assay and accurate clinical examination, were prospectively recorded in a database.
The preoperative staging included the following:
-
Digital examination to evaluate tumor fixation
-
Rigid rectoscopy to obtain macrobiopsies of the tumor, to measure the distance of the lesion from the anal verge, to evaluate the circumferential tumor location, and to select the most appropriate position of the patient on the operating table
-
Total colonoscopy with vital staining and standard endoscopic biopsies at 1 cm around the tumor, with each biopsy identified by a consecutive number and each biopsy site tattooed with india ink
-
EUS with a 7-mHz rotating probe (B&K Company, Herlev, Denmark)
-
MRI, and for the majority of patients, CT imaging, with 3-mm abdominal and pelvic scans
-
Bone scintigraphy and chest x-rays.
Each macrobiopsy was examined by three different morphologists to assess grading. Grading was established based on the degree of cellular differentiation (well [G1], moderately well [G2], or poor [G3]) and on the presence of lymphatic or vessel and neural invasion (Table 1).
Similarly, iT and iN classifications were evaluated by three different radiologists. In case of staging disagreement among the radiologists or between CT imaging, MRI, and EUS, the higher staging was adopted. According to the study protocol, patients who had hypoechogenic lymph nodes with a diameter larger than 8 mm, an irregular aspect, poorly defined margins, and suspicion of metastasis were excluded from local excision.
Radiotherapy
All patients with preoperatively staged iT2 rectal cancer underwent high-dose radiotherapy according to the technique described by Marks et al. [12–14], which comprised a total dose of 50.4 Gy in 28 fractions over 5 weeks. The irradiated areas were the anus, rectum, mesorectum, and regional and iliac lymph-nodes. Since 1997, continuous infusion of 5-FU 200 mg/m2/day as a radiosensitizer has been incorporated into the study protocol [15, 16].
The lesion was evaluated by endoscopy 40 days after the completion of neoadjuvant therapy to determine any reduction in tumor diameter using the tattoo spots as reference points. Staging by EUS, MRI, CT scan, and digital examination also was repeated to identify whether tumor understaging had occurred (postneoadjuvant image staging, yiT, yiN). Patients who had not experienced downstaging were divided into the following three groups according to the downsizing of tumor mass: responders (reduction of more than 50%), low responders (reduction of 50–30%), and nonresponders (<30% reduction or no downsizing). The side effects of radiotherapy were skin erythema for 65% of the patients and diarrhea for 23% of the patients.
Surgery
The operation was performed 45–55 days after radiochemotherapy. Preoperative washout of the colon and short-term antibiotic prophylaxis were used for all the patients. The majority of the patients (94.1%) received TEM under general anesthesia. For eight high-risk patients (5.9%, American Society of Anesthesiology [ASA] score of 4), spinal anesthesia was used according to the decision of the anesthesiologist.
The instrumentation described by Buess and Mentges [17] (Wolf Company, Tuttlingen, Germany) was used in every operation. This included a 12-cm-long modified rectoscope with three-dimensional vision and operative channels. The lesion was preoperatively located with a rigid rectoscope, and the patient consequently was positioned on the operating table in supine, lateral, or prone position to have the lesion located in the inferior part of the visual operative field. The rectoscope was held in position by a Martin arm fixed to the operative table. A working insert with sealing elements to prevent gas loss was connected to the operative rectoscope. Carbon dioxide (CO2) insufflation of the rectum was initiated, and the endoluminal pressure was controlled by the endosurgical unit.
Using monopolar electrocautery, a full-thickness incision of the rectal wall was initiated at a distance of approximately 1 cm around the tumor, beginning on the aboral side (i.e., distal to the tumor or closer to the rectoscope). The incision was carried down to the avascular plane (so-called “holy plane”) for posterior and lateral lesions and at the level of the prostate capsule or vaginal septum for anterior lesions. This incision undermined the margins of rectal wall excision so that the amount of perirectal fat removed together with the tumor was wider than the size of the excised rectal wall.
After the excision, an additional rim of rectal wall was circumferentially removed for intraoperative histology to confirm tumor free margins. The rectal defect then was closed by an endoluminal running suture PDS 00, as described by Buess and Mentges [17].
The patients were examined 1 month after discharge by digital rectal exploration and endoscopy. Subsequent follow-up visits every 6 months included clinical examination, rectoscopy with multiple biopsies, EUS, MRI, or CT scan for the first 3 years, and then annually.
Statistical analysis
Results for continuous variables are presented as median values with 25th to 75th percentiles in parentheses. The study started in May 1992 and ended in March 2008. The cumulative probability of failure (local recurrence or distant metastasis) and the probability of survival were estimated using the Kaplan–Meier method. The 95% confidence interval (95% CI) of curves was based on the Gaussian approximation to the binomial distribution. The SAS System 9.1 (Cary, North Caroline, USA) was used as statistical software for data analysis.
Results
Radiochemotherapy results
Radiochemotherapy downstaged the tumor for 39 of the 84 iT2 patients (24 with ypT0 and 15 with ypT1). Among 45 patients, a tumor volume reduction exceeding 50% was observed in 13 patients (responders) and a mass reduction decrement exceeding 30% in 22 patients (low responders). Only in the remaining 10 patients (11.1%) was no significant response observed (nonresponders) (Table 3).
Short-term results
The median operative time was 95 min (range, 65–120 min.). No intraoperative complications requiring conversion to other surgical procedures were observed. Postoperative pain was minimal, with 12 patients (9%) requiring analgesics (Lixidol 30 mg; Roche S.p.A., Milan, Italy) in the first 48 h. Patients were allowed to drink liquids on the first postoperative day and to eat on the following day. All patients were walking freely within 12 h after the operation. The median hospital stay was 4.5 days (range, 3–8 days).
Minor complications occurred for 12 patients (9%) and included partial leaking suture in 9 patients (6.6%), stool incontinence in 2 patients (1.5%), and rectal hemorrhage in 1 patient (0.7%). Leaking sutures were resolved by 3–5 daily enemas containing antibiotic and analgesic (metronidazole [Bieffe Medital, Grosotto, Italy] 0.5 g/100 ml and lidocaine clorhydrate 200 mg/ml [Bioindustria L.I.M S.p.A., Novi Ligure, Italy]) and occasionally by fasting and parenteral nutrition.
Stool incontinence was treated by physiotherapy and anal sphincter biofeedback, and the symptoms resolved within 2 months of the operation in both cases. The patient with hemorrhage required postoperative transfusion of red blood cells (1 unit).
Major complications were observed in two patients (1.5%). A urethral lesion occurred in a male patient during dissection of a tumor located close to the prostate capsule. The defect was recognized and sutured during the same TEM procedure, and the patient was subsequently discharged with a urinary catheter in place, which was removed 3 weeks later with no untoward sequelae. The second major complication was a perianal phlegmon treated with temporary laparoscopic ileostomy. The median hospital stay was 4.5 days (range, 3–8 days), and the mortality rate was nil.
The correspondence between imaging and definitive histology is reported in Table 2. For the 51 patients preoperatively classified as iT1, definitive histology confirmed pT1 classification of all lesions. Patients who received preoperative radiotherapy (iT2) were classified at definitive histology as ypT0 (n = 24), ypT1 (n = 15), or ypT2 (n = 45) (Table 3).
Long-term results
The median follow-up period was 97 months (range, 55–139 months), and the minimum follow-up period was 28 months. Neither recurrence nor cancer-related mortality was observed in patients with pT0 and pT1 rectal cancer.
Local recurrence occurred in four ypT2 patients (4.7%) after 30, 12, 8, and 6 months, respectively, and all patients underwent laparoscopic abdominal perineal resection (LAPR) (Table 4). In two patients, we observed an extraluminal recurrence. Both patients died of metastatic disease. Only one patient underwent a reoperation (LAPR). The remaining two patients, who had a mucosal recurrence and underwent LAPR, are still alive and disease free at this writing.
An additional two ypT2 patients experienced distant metastases (Table 5). The first patient underwent hepatic resection after 26 months of follow-up evaluation and died 13 months later of systemic disease. The second patient died 24 months later of hepatic and lung metastases.
No local recurrence or distant metastases occurred among patients downstaged after neoadjuvant therapy or among those who showed significant downsizing (≥ 50%) of the tumor. Three of four patients who had a local recurrence and two patients who had experienced distant metastases were classified as nonresponders to neoadjuvant therapy (Tables 4, 5). The remaining patient, with local recurrence, was classified as a low responder.
The patients with preoperatively staged iT1 rectal cancer had no local recurrence and no distant metastases. None died of their disease. The overall survival rate for the iT1 (pT1) patients at the end of the follow-up period (193 months) was 87% (95% CI, 61–96%).
For the patients with preoperative radiotherapy–staged iT2 rectal cancer, the probability of local failure at the end of the follow-up period (193 months) was 5% (95% CI, 2–13%), and the probability of distant metastases was 3% (95% CI, 1–10%) (Table 6). The rectal cancer survival rate at the end of the follow-up period (193 months) was 93% (95% CI, 83–97%), whereas the overall survival at the end of the follow-up period (193 months) for the patients with iT2 rectal cancer was 82% (95% CI, 70–90%).
Discussion
Several goals are important in the management of patients with rectal cancer: local control and prevention of distant metastases, long-term survival, preservation of the anal sphincter, preservation of bladder and sexual functions, and maintenance of quality of life. High complication rates and possible sacrifice of the anal sphincter after radical resection has led clinicians to reconsider the role of local excision in cases of SNALRC.
Local excision of distal rectal cancer has long been used as an alternative surgical option for patients not fit to undergo a major abdominal resection or unwilling to have a permanent stoma. According to several authors [3–5], local excision may be curative for patients with a primary tumor limited in the mucosa. Even if the tumor invades the submucosa, local excision may be potentially curative as long as high-risk features such as poor differentiation, vascular and neural invasion, mucinous histology, and tumor ulceration are not present. The benefits of local excision for these patients are preservation of anal continence as well as bladder and sexual functions, with identical oncologic results [2–5].
Rectal tumors limited to the submucosa (T1) are reported to be at low risk for local recurrence and may therefore be eligible for local excision. In the current series of patients with T1 rectal cancer who underwent TEM, no local recurrence or distant metastases were observed during a median follow-up period of 97 months. Parietal recurrence after local excision may be amenable to surgical salvage, but success depends on early diagnosis.
Once the tumor invades the muscularis propria (T2), preoperative radiochemotherapy is recommended because the available evidence [2, 18–22] shows that local excision alone is associated with high local recurrence rates. In fact, success of local excision in the management of rectal cancer depends on the depth of tumor invasion, and this is correlated with the risk of lymph node metastases [8]. Because traditional local excision of rectal cancer using the Parks technique allows only limited ablation of mesorectal fat adjacent to the tumor, only cancer located in the rectal mucosa is suitable for this approach. Patient selection needs to be based on the probability of positive nodes.
Accurate preoperative assessment of level of invasion is important because the risk of lymph node metastases increases with T stage. The risk is 0–12% for T1, 12–28% for T2, and 36–79 for T3–T4 lesions [18]. Thus, preoperative tumor node metastasis (TNM) classification is a crucial factor for patient selection with curative intent. Preoperative staging should always include EUS combined with new-generation MRI and CT scan.
Digital rectal examination, which still may provide useful information, can no longer be considered an accurate method for staging low and mid rectal cancer because its accuracy depends on the examiner’s experience. A most useful adjunct in preoperative assessment of small rectal lesions is EUS. It provides clear visualization of the layers of the rectal wall, thus enabling accurate evaluation of invasion depth.
Although strongly operator dependent, EUS may be performed with minimal preparation and patient discomfort, and it currently is considered the most accurate method for T classification of SNALRC. The use of EUS has an 82% to 93% accuracy rate for depth of invasion, with overstaging occurring more frequently than downstaging [18]. In assessing lymph node involvement, EUS is less reliable, with an accuracy rate of 65% to 81% [18]. Concerning T classification, a 13% discrepancy rate between EUS and imaging by MRI and CT was observed in the current study.
Patients with rectal cancer undergo abdominal and pelvic MRI before surgery to confirm the iT stage, to evaluate lymph nodes status, and to identify metastatic disease. The accuracy rates for last-generation spiral CT scans (64 MDCT, MultiDetector Computed Tomography) using 3-mm sections are comparable with those for MRI scans in terms of node detection. Both MRI and CT scan give a more accurate evaluation of N status than EUS.
To reduce the risk of false-negatives, restrictive criteria were adopted in the current study. Lymph nodes were considered suspicious for tumor infiltration if they had a diameter larger than 8 mm, were hypoechogenic, or had an irregular shape or undefined margins.
It is important to include at least one radiologist with a specific interest in colorectal disease in the multidisciplinary working group to perform an accurate image staging at the time of the patient’s admittance. In fact, after neoadjuvant therapy, it is difficult to obtain a correct image staging due to edema and tissue sclerosis.
The recent introduction into clinical practice of EUS with fine-needle biopsy in case of suspicious nodes has significantly improved preoperative staging of rectal cancer, and the first experiences with new dyes during MRI are extremely encouraging. Moreover, last-generation CT scan with virtual endoscopy improves the ability to detect neoplasia bulking in the intraperitoneal cavity and provides spatial orientation of the neoplasia.
Undoubtedly, the more recent advancements in imaging techniques (EUS, MRI, and CT) have achieved a remarkably high incidence of correct diagnosis. In the authors’ experience, the risk of understaging is less than 5%. In case of understaging identified at definitive histology, traditional resection with TME can subsequently be performed.
For patients with T2 cancer, local recurrence after traditional local excision alone is unacceptable, but by combining radiochemotherapy with the TEM technique, it is possible to improve local control and treat the patient with a minimally invasive approach also in the case of small, highly selected T2 rectal cancer.
Preoperative radiotherapy reduces the local recurrence rate. It offers the opportunity for tumor downstaging and improves quality of life by preservation of the anal sphincter [21–25]. In the current study, which included selected patients with small T2 rectal cancer undergoing preoperative radiochemotherapy followed by TEM, four patients (4.7%) had a local recurrence, and two patients (2.3%) experienced distant metastases. If the entire group of patients with T1 and T2 (N0) rectal cancer is considered, the local recurrence and distant metastases rates are 2.9% and 1.4%, respectively. Disease-free survival at the end of the follow-up period is 93%.
The favorable results for local excision in the current series are essentially related to five main factors:
-
1.
The integrated activities of a team with specific experience. The oncologist, radiologist, pathologist, endoscopist, and radiotherapist all are just as important as the surgeon in this multidisciplinary team.
-
2.
Correct image staging obtained before neoadjuvant therapy. This staging is crucial. Each T and N classification by EUS, MRI, and CT results from evaluation by three different operators. It is on the basis of this evaluation that three crucial decisions are made: the indication to perform radiotherapy, the evaluation of the response to neoadjuvant treatment, and whether to treat the patient with TEM or not.
-
3.
Radiotherapy. Notably, high-dose radiotherapy causes significant modifications in tumor morphology of the surgical specimen that make correct evaluation of tumor grading difficult. To obtain a correct grading classification, macrobiopsies of the tumor must be taken before neoadjuvant treatment. Quality standards and clinical management of radiotherapy are too often underestimated. Downstaging, downsizing, and the tumor regression score (TRC) according to the criteria of Mandard et al. [26] are the most reliable prognostic predictors. The current study confirms the authors’ previous observation [2] that the response to neoadjuvant therapy is the best predictive factor of local recurrence and distant metastases. Low responders and nonresponders to neoadjuvant therapy had the worst result. For this reason, local excision currently is withheld for nonresponders except in case of palliation.
-
4.
Correct technique of an “adequate” local excision. This factor is very important, as for TME by traditional surgery. Published studies on local excision too often do not specify the methods for evaluation if local excision was adequate in terms of circumferential margin and radial clearance from the tumor. Compared with Park’s technique, TEM provides excellent exposure of the operative field with a stereoscopic magnified endoscopic vision that allows extremely precise dissection and wide excision of both the rectal wall and adjacent mesorectum with appropriate and oncologically correct margins. To remove an adequate margin of normal mucosa around the tumor, vital staining of the rectal mucosa during endoscopy is routinely performed. In fact, small polyps or flat adenomas are frequently found around the main lesion, which may be responsible for both malignant and benign local recurrence. Vital staining should not be performed during TEM because the blue dye reduces vision of the operative field. Furthermore, before neoadjuvant treatment, six to eight biopsies of apparently normal mucosa 1 cm around the tumor are strongly recommended, with an india ink tattoo at each biopsy site to define the exact limits of the normal mucosa. Once the specimen is obtained, a further full-thickness slice of rectal wall is removed from both the oral and aboral margins of excision to avoid the risk of having both positive and false-positive margins of the specimen. False-positive margins can occur occasionally when the specimen safety margins are fissured due to the intraoperative manipulations.
-
5.
The amount of mesorectum removed together with the rectal wall is another relevant factor that differentiates the current approach from that of other authors. In fact, the surgical technique of local excision has been standardized for removal of not only the rectal wall including the tumor but also the adjacent mesorectum. In case of posterior or posterolateral location of the tumor, the dissection follows the so called “holy plane” as in TME, extending the dissection laterally to include as much perirectal fat as possible. Once the specimen is removed, the pelvic floor and the levator ani muscles should appear through the parietal defect, completely free of any fat. For tumors located on the anterior wall of the rectum, the prostate capsula or the rectal-vaginal septum are the correct planes of deep dissection. Independent of tumor location, mesorectal dissection is widely extended well below the oral, aboral, and lateral limits of mucosa incision to reduce tension on the suture line. At the end of dissection, the removed specimen should have the shape of a truncated pyramid, with the neoplasia and surrounding normal mucosa at the top and the mesorectal fascia at the bottom. In the authors’ experience, such wide and precise dissection may be achieved only with the TEM instrumentation.
Recently, a modified perioperative lymphoscintigraphy has been developed that currently is included routinely in the protocol to improve the intraoperative lymphatic tissue harvest. The results of this study will be the object of another report.
In conclusion, the current findings confirm that local excision of T1 rectal cancer avoids major surgery or colostomy without compromising the oncologic results. If the tumor invades the muscularis propria (T2), neoadjuvant treatment using high-dose radiation combined with adequate full-thickness local excision shows the same oncologic results as TME, and it appears to be a promising option also in the management of highly selected patients with small T2 rectal cancer. This minimally invasive approach minimizes the risks of postoperative morbidity and mortality as well as the need for stomas that may be associated with traditional surgery. The hospital stay also is remarkably reduced, and the quality of life is improved, as recently reported in a prospective randomized trial comparing TEM with laparoscopic TME for T2N0 small rectal tumors [25]. In the authors’ opinion, these benefits may be achieved by close collaboration within a multidisciplinary team, use of neoadjuvant therapy for all T2 tumors, correct image staging and careful patient selection, and an adequate local excision by the TEM technique.
References
Franklin ME, Kazantsev GB, Abrego D, Diaz-E JA, Balli J, Glass JL (2000) Laparoscopic surgery for stage III colon cancer. Surg Endosc 14:612–616
Lezoche E, Guerrieri M, Paganini AM, Baldarelli M, De Sanctis A, Lezoche G (2005) Long-term results in patients with T2–3 N0 distal rectal cancer undergoing radiotherapy before transanal endoscopic microsurgery. Br J Surg 92:1546–1552
Blair S, Ellenhorn JD (2000) Transanal excision for low rectal cancers is curative in early-stage disease with favorable histology. Am Surg 66:817–820
Winde G, Nottberg H, Keller R, Schmid KW, Bunte H (1996) Surgical cure for early rectal carcinomas (T1): transanal endoscopic microsurgery vs anterior resection. Dis Colon Rectum 39:969–976
Sengupta S, Tjandra JJ (2001) Local excision of rectal cancer: what is the evidence? Dis Colon Rectum 44:1345–1361
Heintz A, Morschel M, Junginger T (1998) Comparison of results after transanal endoscopic microsurgery and radical resection for T1 carcinoma of the rectum. Surg Endosc 12:1145–1148
Kim CJ, Yeatman TJ, Coppola D, Trotti A, Williams B, Barthel JS, Dinwoodie W, Karl RC, Marcet J (2001) Local excision of T2 and T3 rectal cancers after downstaging chemoradiation. Ann Surg 234:352–358 discussion 358–359
Bozzetto F, Baratti D, Andreola S, Zucali R, Schiavo M, Spinelli P, Gronchi A, Bertario L, Mariani L, Gennari L (1999) Preoperative radiation therapy for patients with T2–T3 carcinoma of the middle-to-lower rectum. Cancer 86:398–404
Rouanet P, Fabre JM, Dubois JB, Dravet F, Saint Aubert B, Pradel J, Ychou M, Solassol C, Pujol H (1995) Conservative surgery for low rectal carcinoma after high-dose radiation. Ann Surg 221:67–73
Habr-Gama A, Perez RO, Kiss DR, Rawet V, Scanavini A, Santinho PM, Nadalin W (2004) Preoperative chemoradiation therapy for low rectal cancer: impact on downstaging and sphincter-saving operations. Hepatogastroenterology 51:1703–1707
Lee W, Lee D, Choi S, Chun H (2003) Transanal endoscopic microsurgery and radical surgery for T1 and T2 rectal cancer. Surg Endosc 17:1283–1287
Marks G, Mohiuddin M, Masoni L (1993) The reality of radical sphincter preservation surgery for rectal of the distal 3 cm of rectum following high-dose radiation. Int J Radiat Oncol Biol Phys 27:779–783
Marks G, Mohuiddin M, Rakinic J (1991) New hope and promise for sphincter preservation in the management of cancer of the recum. Semin Oncol 18:88–98
Marks G, Mohuiddin M, Masoni L, Pecchioli L (1990) High-dose preoperative radiation and full-thickness local excision: a new option for patients with select cancers of the rectum. Dis Colon Rectum 33:735–739
Valentini V, Coco C, Picciocchi A, Morganti AG, Trodella L, Ciabattoni A, Cellini F, Barbaro B, Cogliandolo S, Nuzzo G, Doglietto GB, Ambesi-Impiombato F, Cosimelli M (2002) Does downstaging predict improved outcome after preoperative chemoradiation for extraperitoneal locally advanced rectal cancer? A long term of 165 patients. Int J Radiat Oncol Biol Phys 53:664–674
Crane CH, Skibber JM, Birnbaum EH (2003) The addition of continuous infusion 5-FU to preoperative radiation therapy increases tumor response, leading to increased sphincter reservation in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 57:84–89
Buess G, Mentges B (1992) Transanal endoscopic microsurgery (TEM). Minim Invasive Ther 1:101–109
Varma MG, Rogers SJ, Schrock TR, Welton ML (1999) Local excision of rectal carcinoma. Arch Surg 134:863–867
Balch GC, De Meo A, Guillem JG (2006) Modern management of rectal cancer: a 2006 update. World J Gastroenterol 12:3186–3195
Marijnem CA, Glimelius B (2002) The role of radiotherapy in rectal cancer. Eur J Cancer 38:943–952
Martling AL, Holm T, Rutqvist LE, Moran BJ, Heald RJ, Cedemark B (2000) Effect of surgical training programme on outcome of rectal cancer in the County of Stockholm. Stockholm Colorectal Cancer Study Group, Basingstoke Bowel Cancer Research Project. Lancet 356:93–96
Rodel C, Martus P, Papadoupolos T, Fuzesi L, Klimpfinger M, Fietkau R, Hohenberger W, Raab R, Sauer R, Wittekind C (2005) Prognostic significance of tumor regression after chemoradiotherapy for rectal cancer. J Clin Oncol 23:8688–8696
Ruo L, Guillem JG, Minsky BD, Quan SH, Paty PB, Cohen AM (2002) Preoperative radiation with or without chemotherapy and full-thickness transanal excision for selected T2 and T3 distal rectal cancers. Int J Colorectal Dis 17:54–58
Vecchio FM, Valentini V, Minsky BD, Padula GD, Venkatraman ES, Balducci M, Micciche F, Ricci R, Morgani AG, Gambacorta MA, Maurizi F, Coco C (2005) The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncl Biol Phys 62:752–760
Lezoche G, Baldarelli M, Guerrieri M, Paganini AM, De Sanctis A, Bartolacci S, Lezoche E (2008) A prospective randomized study with a 5 years minimum follow-up of transanal endoscopic microsurgery versus laparoscopic total mesorectal excision after neoadjuvant therapy. Surg Endosc 22:352–358
Mandard AM, Dalibard F, Mandard A (1994) Pathological assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Cancer 73:2680–2686
Disclosures
Giovanni Lezoche, Mario Guerrieri, Maddalena Baldarelli, Alessandro Maria Paganini, Giancarlo D’Ambrosio, Roberto Campagnacci, Silvia Bartolacci, and Emanuele Lezoche have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lezoche, G., Guerrieri, M., Baldarelli, M. et al. Transanal endoscopic microsurgery for 135 patients with small nonadvanced low rectal cancer (iT1–iT2, iN0): short- and long-term results. Surg Endosc 25, 1222–1229 (2011). https://doi.org/10.1007/s00464-010-1347-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-010-1347-9